Chapter 4 ... 1. Identify each of the following as atoms or...
advertisement

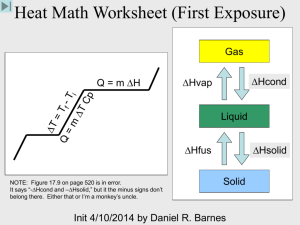
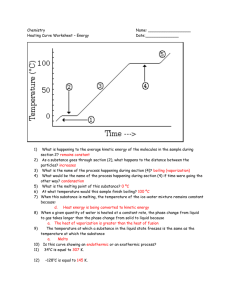
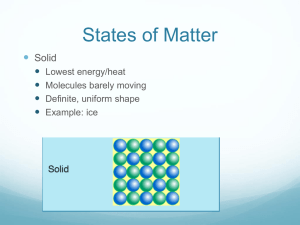
Chapter 4 Review Exercises PSC1515 1. Identify each of the following as atoms or molecules and as elements or compounds: a. Iron (Fe) __________ _____________ b. carbon dioxide (CO2) __________ _____________ c. nitrogen (N2) __________ _____________ 2. What movement exist for particles in each of the following states of matter? a. solid _______________________________ b. liquid_______________________________ c. gas________________________________ 3. What is the relationship between heat, temperature, kinetic energy and speed of movement of particles in a substance? As more heat is applied to a substance, the temperature ______________, the kinetic energy_____________and the speed_________________. 4. Convert the following: a. 45oC to oF b. -67oF to oC c. -210oC to K 5. The freezing point of water is _________oC, ____________oF or ____________K. 6. The boiling point of water is _________oC, ____________oF or ____________K. 7. The magnitude of 1oC is equal to ______K and _______oF. 8. Absolute zero is _______K. 9. Identify each of the following as heating or cooling: a. Internal energy decreases______________ b. Internal energy increases_______________ 10. 1 cal=_______J 1 kcal = ________ cal 11. The specific heat of substance A is .657 cal/goC. If 27 kcal of heat are applied to equal masses of water and of substance A, which of the two substances will increase more in temperature?____________ 12. What is the specific heat of a substance in J/goC if 6.9 grams of it increase in temperature by 12.2oC after the application of 43.7 J? 13. How much heat is required in calories to increase the temperature of 578 grams of water from 20oC to 87oC? 14. Identify the following changes in physical state: a. sugar becoming syrup____________________ b. dry ice converting to carbon dioxide gas___________________ c. water vapor transforming to dew___________________ d. rain becoming snow____________________ e. a puddle of water disappearing after the sun shines on it________________________ 15. Another term for melting is _____________. Vaporization can be _________________ (below the bp) or ___________________(at the boiling point). 16. Match the law of thermodynamics with the description: First, second or third law The entropy of the universe is always increasing. _________ The work done on a system is equal to the internal energy of the system plus the work done by the system.__________ Energy can neither be created nor destroyed, only converted from one form to another.__________ All movement stops at absolute zero.___________ 17. In the diagram below what do each of the letters represent? D T E M P E B C A Heat 1. a. atom, element b. molecule, compound c. molecule, compound 2. a. vibration b. vibration and rotation c. vibration, rotation and translation 3. increases, increases, increases 4. a. 113oF b. -55oF c. 63K 5. 0oC, 32oF, 273K 6. 100oC, 212oF, 373K 7. 1K and 1.8oF 8. 0K 9. a. cooling b. heating 10. 4.184, 1000 11. Substance A 12. .519 J/goC 13. 38,726 cal 14. a. melting b. subliming c. condensing d. freezing e. evaporating 15. fusion, evaporation, boiling 16. a. second b. first c. first d. third 17. A) solid heats B) melting C) liquid heats D) vaporization E) vapor heats A____________ B____________ C____________ D____________ E____________ A