Diffusion Tractography as a guide for functional neurosurgery
advertisement

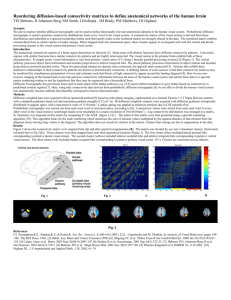
Diffusion Tractography as a guide for functional neurosurgery Johansen-Berg H1, Behrens TEJ1, Stein JF2, Aziz TZ2,3 1Centre for Functional MRI of the Brain, University of Oxford, UK; 2Dept of Physiology, University of Oxford, UK, Dept of Neurosurgery, Radcliffe Infirmary, Oxford Introduction Phase 2: Define surgical targets based on connection patterns • Deep Brain Stimulation can be an effective treatment for movement, pain, mood, and anxiety disorder Chronic Pain • Periacquaductal-periventricular gray, and nucleus cuneiformis are popular targets for treatment of pain disorders. • Accurate target localisation is critical for efficacy but challenging using conventional approaches • We have provided the first descriptions of the anatomical connections of these areas in the human brain using DTI [4-6] • Effects of stimulation are probably mediated via anatomical connections from the stimulation site • Both structures connect strongly to thalamus and prefrontal cortex, providing routes by which DBS could influence the pain matrix • Diffusion-weighted MRI and tractography provides a method for non-invasive visualisation of anatomical connections We aimed to test whether diffusion MRI and tractography could aid targeting by identifying structures based on their anatomical connections. Phase 1: Characterise variability reproducibility in the healthy population and Variability in tractography and diffusion measures Quantitative diffusion measures, such as fractional anisotropy (FA), are sensitive to changes in white matter microstructure. If these measures are to be used to address clinical questions it is important to characterise their variability. We scanned 8 subjects on 3 different days to quantify between subject and between session variation in tractography results using a range of methods Overlap across subjects Overlap across sessions Movement Disorders • The pedunculopontine and subthalamic nuclei are important targets for treatment of movement disorders • We have characterised the connections of these regions in the human brain using tractography [7] • This includes precise descriptions of the topography of connections with different cortical and subcortical areas as well as somatotopic mapping to different body part representations in M1. Depression a) c) d) e) f) b) Steps involved in blind parcellation. Probabilistic tractography is run from every voxel in a seed mask (a) and the probability of connection to all other voxels in the brain (b) is recorded in a connectivity matrix (c). The cross-correlation matrix (d) of the connectivity matrix is found and re-ordered (e) to bring voxels with similar connection patterns close to each other. Clusters in the re-ordered matrix represent regions with distinct connection patterns. This approach reliably differentiates SMA/pre-SMA based on connectivity (f, top), and these regions correspond closely to those defined based on function using FMRI (f, bottom). From ref [2] Day 1 Spectral reordering Day 4 - 3T Ideal segmentation Day 3 Day 2 Individual anatomy k-means clus t ering Using the data from 8 subjects scanned 3 times at 1.5T and once at 3T we tested the reproducibility of the approach: • Overlap between days 89.3% • Overlap between scanners 87.3% • Overlap with FMRI-based parcellations 80% • Overlap with cytoarchitectonic maps 93% See ref [3] A. Location of seed points in STN on coronal slice . B. Schematic of STN, showing seed voxels. C. Topography of cortical connections. D. Zoomed version of topography. Consistent with animal data, cortical motor areas (M1, SMA, PMC) connect to dorso-lateral portions of STN whereas connections to associative subcortical areas are found in more ventral portions • DBS of the white matter underlying the subgenual cingulate results in dramatic remission of symptoms in some previously drug-resistant patients • We have used blind cortical parcellation to define subregions within subgenual cingulate and have defined the connections of these regions (right) For measures of mean fractional anisotropy (FA) along tracts, co-efficients of variation (CV) were below 5% between sessions and below 10% between subjects. These variability measures were used to perform power calculations to determine numbers of subjects required to detect differences of a given size between groups of over time [1]. Variability in ‘Blind’ Cortical Parcellation We previously proposed a method for parcellation of grey matter areas based on their pattern of anatomical connections [2]. This approach could aid targeting but first it is important to establish its reliability and validity. Tracts from PVG/PAG Top: Location of electrode contact points (red=responders; blue=non-responders) in relation to anterior (red-yellow) and posterior (blue-turquoise) clusters. Bottom: Paths from effective electrode contacts consistently travel to amygdala, nucleus accumbens, hypothalamus, cingulum bundle, medial frontal cortex and orbitofrontal cortex. After re-ordering of x-correlation matrices, each matrix was manually divided into two clusters, which are mapped back onto the brain. Data for 16 healthy subjects are shown. Group average paths from posterior cluster (top, blue) and anterior cluster (bottom, red-yellow) • In collaboration with Helen Mayberg (Emory, USA), we have projected electrode locations onto DTI data to define the network of connections that potentially mediates therapeutic effects [8] Discussion • We have characterised the anatomical connections from surgical targets of interest in movement, pain, and mood disorders. • An outstanding question is the relationship between electrode placement and outcome. Specifically, we aim to show that the pattern of anatomical connections of a target site will partly determine surgical efficacy. • We are therefore also acquiring pre-operative DTI data in patients themselves so that we can test the connections from electrode locations using the individual’s own brain anatomy and relate this information to clinical outcome References: 1. Heiervang, Behrens, Mackay, Robson, Johansen-Berg. Between session reproducibility and between subject variability of diffusion MR and tractography measures. NeuroImage. In press. 2. Johansen-Berg, Behrens, Robson, Drobnjak, Rushworth, Brady, Smith and Matthews. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proceedings of the National Academy of Sciences USA. 101. 1333540. 2004. 3. Klein, Behrens, Robson, Mackay, Higham and Johansen-Berg. Connectivity-based parcellation of human cortex using diffusion MRI: establishing reproducibility, validity and observer-independence in BA 44/45 and SMA/pre-SMA. NeuroImage. In press. 4. Sillery, Bittar, Robson, Behrens, Aziz, Stein and Johansen-Berg (2005) Connectivity of the human periventricular gray. Journal of Neurosurgery. 103(6):1030-4. 5. Owen, Green, Davies, Stein, Aziz, Behrens, Voets, Johansen-Berg. Connectivity of an effective hypothalamic surgical target for cluster headache. Journal of Clinical Neuroscience. In press. 6. Hadjipavlou, Dunckley, Behrens and Tracey. Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: a diffusion tensor imaging study in healthy controls. Pain. 123. 169-178. 7. Aravamuthan, Arasu, Johansen-Berg, Voets, Liu, Stein, Aziz. Human pedunculopontine nucleus connections traced using probabilistic diffusion tractography. Society for Neuroscience Abstracts 2006. 8. Johansen-Berg, Behrens, Matthews, Katz, Metwalli, Lozano and Mayberg. Connectivtiy of a subgenual cingulate target for treatment resistant depression. OHBM Meeting , 2006