PHL 315 cholinergic
advertisement

PHL 315
Pharmacology I
Part II
Naglaa F. El-Orabi, Ph D, M Sc, B SC
Department of Pharmacology & Toxicology,
College of Pharmacy,
King Saud University, Riyadh, KSA
Cholinergic
Transmission
References
“ Rang & Dale’s Pharmacology”
6th ed.,
Rang HP, Dale MM, Ritter JM, Moore PK, eds.
Elsevier Science, 2007. Chapter 10, page 144
Cholinergic transmission
• An important traditional classification of autonomic
nerves is based on the primary neurotransmitter molecules
released from their terminals into adrenergic and
cholinergic.
• A large number of peripheral ANS fibers synthesize and
release Ach; they are cholinergic fibers.
• These include all preganglionic efferent autonomic
fibers (both sympathetic and parasympathetic).
• In addition, most parasympathetic
and some
sympathetic (to sweat glands) postganglionic fibers are
cholinergic.
• Somatic (non-autonomic) motor fibers (NMJs) to skeletal
muscle (motor end plates).
• Ach is also very important central NT.
Parasympathetic nervous syste (PSNS)
Anatomy
• PSNS originates from cranio-sacral parts of
spinal cord.
• Cranial outflow originate from cranial nerve
nuclei in the brain stem. Preganglionic fibers
run via:
a)Oculomotor nerve (III)
b)Facial nerve (VII)
c)Glossopharyngeal nerve (IX)
d)Vagus nerve (X)
• These nerve fibers innervate organs of the head
& neck (eye, nasal mucosa, salivary glands,…),
thorax & upper abdomen
( heart,
respiratory system, esophagus, stomach,
pancreas, liver, small intestine and upper half
of the large intestine).
PSNS anatomy (cont.)
• Sacral outflow originate from visceral motor region of spinal
cord (S2-S4). Preganglionic fibers run via pelvic nerves.
• These nerve fibers innervate organs of the pelvis and lower
abdomen (lower half of large intestine, the rectum, urinary and
reproductive systems)
• PSNS does not innervate most of blood vessels, sweat glands,
adrenal medulla and arrector pili muscles.
PSNS anatomy (cont.)
• Parasympathetic pathway
• Brain areas (hypothalamus & brain stem)
• Cranial or sacral outflow
• Relatively long pre-ganglionic neurons to terminal or intramural
ganglia (in walls of viscera, close to effector organs)
• Short post-ganglionic neurons.
PSNS
Neurotransmitters
• All
Parasympathetic
nerve
fiber
(both
preganglionic
postganglionic) are cholinergic, that is, they work by releasing
ACh neurotransmitter.
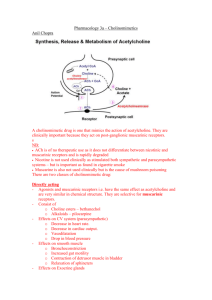
• Acetylcholine is synthesized in the cytoplasm of neuronal cells
from acetyl-CoA and choline through the catalytic action of the
enzyme choline acetyltransferase (ChAT).
• Acetylcholine is then transported into the storage vesicle. Release of
transmitter occurs when voltage-sensitive calcium channels in the
terminal membrane are opened, allowing an influx of Ca2+. The
resulting increase in intracellular Ca2+ causes fusion of vesicles with
the surface membrane and exocytotic expulsion of acetylcholine
into the junctional cleft and interact with postsynaptic receptors.
PSNS neurotransmitters (cont.)
• Acetylcholine's action is terminated by metabolic degradation
by the enzyme acetylcholinesterase (AChE). AChE splits ACh
into choline and acetate, neither of which has significant
transmitter effect, and thereby terminates the action of the
transmitter . Most cholinergic synapses are richly supplied with
AChEs; the half-life of ACh in the synapse is very short (1-2
milliseconds).
• AChE is also found in other tissues, eg, RBCs. Another
cholinesterase
with
a
lower
specificity
for
ACh,
butyrylcholinesterase (pseudocholinesterase) , is found in
blood plasma, liver, glia, and many other tissues. Little or no
acetylcholine escapes into the circulation. Any acetylcholine that
reaches the circulation is immediately inactivated by plasma
esterases.
ChAT
ACETYLCHOLINE
VAT
Na+- CHT
Cholinergic receptors
•Cholinergic receptors have two major families;
(nAChR) and muscarinic (mAChR) receptors.
nicotinic
•Ach acts as specific agonist for both receptor subclass
•In contrast, because of their unique configurations,
Nicotine and Muscarine are selective for the cholinergic
receptor subtypes whose structure complements their own.
(a) Muscarinic receptors
• mAChRs are G-protein-coupled receptors
causing:
• activation of PLC (hence ↑IP3, DAG as
2nd messengers)
• inhibition of adenylyl cyclase (↓cAMP)
• activation or inhibition of ion (K+ & Ca2+
) channels .
• mAChRs
mediate
ACh
effects
at
postganglionic parasympathetic synapses
(mainly heart, smooth muscle, glands),
and contribute to ganglionic excitation.
They occur in many parts of the CNS.
• Five main types of mAChR occur (M1-5).
• All mAChRs are activated by acetylcholine
and blocked by atropine. There are also
subtype-selective agonists and antagonists.
Muscarinic receptors (cont.)
Receptor
type
Location
MOA
Functional
response
Stimulatory (↑IP3, DAG,
↑intracellualr Ca2+)
CNS excitation (?memory)
Gastric secretion
(neuronal)
Autonomic ganglia,
Glands ,
CNS (cerebral cortex)
M2
Myocardium, smooth
muscles, some in CNS
Inhibitory (↓cAMP, ↓K+ & Ca2+
conductance)
Cardiac inhibition
Neural inhibition
Central muscarinic effects (e.g.
tremor, hypothermia)
Exocrine glands ,
Smooth muscle (GIT,
eye, airways, bladder)
Vessels endothelium,
Stimulatory (↑IP3,
↑intracellualr Ca2+)
Gastric, salivary secretion GI
smooth muscle contraction
Ocular accommodation
Vasodilatation
CNS
Inhibitory (↓cAMP)
Enhanced locomotion
CNS: very localised in
substantia nigra,
Salivary glands, Eye
(Iris/ciliary muscle)
Stimulatory (↑IP3 Excitation)
Not known
M1
(cardiac)
M3
(glandular ,
smooth muscles)
M4
M5
(b) Nicotinic receptors
• nAChRs are directly coupled to
cation channels (like Na+/K+
channels), and mediate fast
excitatory synaptic transmission
at the neuromuscular junction
(Skeletal muscles), autonomic
ganglia, and various sites in CNS.)
• Muscle (Nm) and neuronal (Nn)
nAChRs differ in their molecular
structure and pharmacology.
• mAChRs and nAChRs occur
presynaptically
as
well
as
postsynaptically, and function to
regulate transmitter release.
Nicotinic receptors (cont.)
Receptor
type
Location
MOA
Functional
response
Nn
Post
ganglionic
neurons,
adrenal
medulla
Opening of Na+, K+
channels,
depolarization
Excitation of autonomic
ganglia
Stimulate Epi, NE from
adrenal gland
Nm
Skeletal
muscle
neuromusc
ular
endplates
Opening of Na+, K+
channels,
depolarization
Skeletal muscle contraction
(α1)2β1δγ
Physiological actions of muscarinic stimulation
Organ
Eye
Heart
Blood vessels
GIT
Receptor
Action
Circular
muscle of the
iris
M3
Contracts(miosis)
Ciliary muscle
M3
Contracts
SA node
M2
Slows
Myocardium
(Atrium
&
ventricles)
M2
Negative inotropic (Reduced
contractility) action (more in
atria)
and
negative
chronotropic action
AV node
M2
Reduced conduction velocity
Endothelium
M3
Vasodilatation
Smooth
muscle walls
M3
Contraction ( motility)
Sphincters
M3
Relax
Glands
M3
Secretion
Gallbladder
M3
Contraction
Physiological actions of muscarinic stimulation
(cont.)
Organ
Receptor
Action
Smooth
muscles
M3
Contraction
Glands
M3
Secretion
Wall
(detrusor)
M3
Contracts
Trigone
sphincter
M3
Relax
Pregnant uterus
M3
Contracts
Penis and clitoris
M3
Erection
Glands (Salivary, Lacrimal,
Nasopharyngeal ,
vaginal lubrication&
Sweat ; symp.
Cholinergic)
M3
Secretion
Bronchi
Urinary bladder
Modifying Autonomic Nervous System Function
Parasympathomimetics = Cholinoceptor stimulants: bind to
acetylcholine receptors (Muscarinic & Nicotinic) and stimulates
them or enhance cholinergic transmission by other mechanism:
Muscarinic agonists (stimulants)
Anticholinesterases and other drugs that enhance cholinergic
transmission.
Ganglion-stimulating drugs
Parasympatholytics (cholinergic antagonists – anticholinergic
drugs): bind to acetylcholine receptors and reduce the effects of
parasympathetic
stimulation
by
preventing
endogenous
acetylcholine from binding to them:
Muscarinic antagonists
ganglion-blocking drugs
Neuromuscular-blocking drugs
ChAT
ACETYLCHOLINE
ACETYLCHOLINE
VAT
Na+- CHT
Muscarinic
antagonists
Parasympathomimetics
(Muscarinic receptor stimulants)
Choline Esters
Direct
Cholinomimetic
alkaloids
Cholinoceptor
stimulants
Indirect
Cholinesterase
inhibitors
Direct cholinoceptor
stimulants
Choline esters
e.g. Acetylcholine, Methacholine, Carbachol and
Bethanechol
Colinomimetic alkaloids
e.g., Muscarine, Oxotremorine and Pilocarpine
• Many of these muscarinic agonists are used as
experemintal tools.
• Use of muscarinic receptor agonists, is
contraindicated in patients with asthma, coronary
insufficiency and peptic ulcers
Direct cholinoceptor stimulants (cont.)
1- Bethanechol (Urecholine®)
• Selectively stimulates muscarinic receptors
(with further selectivity for M3 receptors)
• Unlike acetylcholine, bethanechol is not
hydrolyzed by cholinesterase and will therefore
have a long duration of action
• Clinical uses:
1.
To assist bladder emptying in non-obstructive urinary
retention resulting from general anesthetic or diabetic
neuropathy of the bladder
2. To treat gastroparesis (delayed gastric emptying), because it
stimulates GI motility and secretion
3. To stimulate salivary gland secretion in patients with
xerostomia (dry mouth, nasal passages, and throat)
Bethanechol (cont.)
Side Effects associated with bethanechol
therapy:
1.Abdominal cramps or discomfort
2.Nausea and diarrhea
3.Excessive salivation
4.Hypotension and bradycardia
5.Urinary urgency
6.Bronchial constriction and asthmatic
attacks
Direct cholinoceptor
stimulants (cont.)
1- Pilocarpine (Salagen®)
Indications: It is more commonly used than
bethanechol to induce salivation, and also
for various purposes in ophthalmology.
1. Treatment of primary or acute
glaucoma and also to lower IOP prior
to surgery for acute glaucoma by local
instillation in the form of eye drops.
2. Treatment of symptoms of dry mouth
from salivary gland hypofunction
caused by radiotherapy for cancer of the
head and neck (xerostomia )
Pilocarpine(cont.)
Side Effects associated with pilocarpine
therapy:
Most of them are related to its non-selective action as a
muscarinic receptor agonist
1.Excessive sweating
2.Excessive salivation
3.Bronchospasm and increased bronchial mucus secretion
4.Bradycardia, hypotension
5.Nausea and diarrhea
6.It may result in miosis when used chronically as an eye
drop
Muscarinic effects on the eye
Normal:
Ciliary Muscle Relaxed
Suspensory Ligaments Under Tension
Lens is Flattened
Focus on Distant Objects
Accommodation:
Ciliary Muscle Contracts
Reduced Tension on Suspensory Ligaments
Lens becomes Round
Focus on Near Objects
Muscarinic effects on the eye (cont.)
• The smooth muscles of the iris:
• The sphincter muscle is innervated
by M3 receptors. Its contraction
under the influence of muscarinic
agonist (e.g. pilocarpine) results in
miosis, and its blockade by
muscarinic antagonist (e.g. atropine)
results in mydriasis.
• On the other hand, the radial
muscle is innervated by α-1 receptor.
Its contraction by an agonist results
in mydriasis and its blockade results
in miosis.
Sphincter
muscle
Radial
muscle
Glaucoma
• Glaucoma is an eye disorder in which the optic
nerve suffers damage, permanently impacting vision
in the affected eye(s) and progressing to complete
blindness if untreated.
• It is often associated with increased pressure of the
aqueous humour in the eye (Intraocular pressure
“IOP”).
• The aqueous humour is a thick watery substance
filling the space between the lens and the cornea. It
is rich in amino acid, glucose, antioxidants , and
immunoglobulins. Its main role to maintains IOP
and keep the eyes slightly distended. In addition to
providing nutrition and protection for the occular
tissues
• Aqueous humour is secreted into the posterior
chamber by the ciliary body epithelium, it flows in
through the pupil to the anterior chamber, and then
to drain out of the eye via Schlemm's canal into the
veins of the orbit.
Glaucoma (cont.)
Drug
Ecothiopate,
Pilocarpine,
Mechanism
Cholinomimetics
Physostigmine
Timolol,
carteolol
β-Adrenoceptor
antagonist
Acetazolamide
dorzolamide
Carbonic
anhydrase
inhibitor
Brimonidine
Clonidine,
apraclonidine
Pratanoprost,
Travoprost
α2-Adrenoceptor
agonist
Prostaglandin
analogue
Notes
work by contraction of the iris sphinctr muscle
(miosis) and ciliary muscl that tightening the
trabecular meshwork and allowing increased
outflow of the aqueous humour. Widely used as eye
drops. Can cause muscle spasm and systemic effects.
decrease aqueous humor production by the ciliary
body epithelium. Given locally as eye drops but may
still cause systemic side effects: bradycardia,
bronchoconstriction.
lower secretion of aqueous humor by inhibiting
carbonic anhydrase in the ciliary body epithelium
Acetazolamide is given systemically. Side effects
include diuresis, loss of appetite, tingling,
neutropenia.
Dorzolamide is used as eye drops. Side effects
include bitter taste and burning sensation.
work by a dual mechanism, decreasing aqueous
humor production and increasing trabecular
outflow. Used locally as eye drops
Increase aqueous humor outflow. Given locally as
eye drops. Can cause ocular pigmentation
Parasympathomimetics
(Muscarinic receptor
stimulants)
Choline
Esters
Direct
Cholinomimetic
alkaloids
Cholinoceptor
stimulants
Indirect
Cholinesterase
inhibitors
Indirect cholinoceptor stimulants
• Drugs that enhance cholinergic transmission
act either by inhibiting cholinesterase or by
increasing ACh release.
• Example of drugs that enhance cholinergic
transmission via increase of Ach release:
- Aminopyridines, which block K+ channels
and thus prolong the action potential in the
presynaptic nerve terminal.
- This drug are not selective for cholinergic nerves
but increase the evoked release of many
different transmitters, so have too many
unwanted effects to be useful in treating
neuromuscular disorders.
Cholinesterase inhibitors
• Indirect-acting agents produce their primary effects by
inhibiting acetylcholinesterase, which hydrolyzes
acetylcholine to choline and acetic acid by forming a
complex with acetylcholinesterase enzyme .By inhibiting
acetylcholinesterase, the indirect-acting drugs increase
the endogenous ACh concentration in synaptic clefts and
neuroeffector junctions. The excess ACh, in turn,
stimulates cholinergic receptors to evoke increased
responses. These drugs act primarily where ACh is
physiologically released and are thus amplifiers of
endogenous ACh.
• Some
cholinesterase
inhibitors
also
inhibit
butyrylcholinesterase (pseudocholinesterase).
Cholinesterase inhibitors (Cont’d)
• The inhibitory effect of anticholinesterases
may be:
• Reversible:
as that produced by
edrophonium,
pyridostigmine,
physostigmine (eserin) or neostigmine
• Irreversible: such as echothiophate and
malathion
(orgonophosphorus
compounds).
Cholinesterase inhibitors (Cont’d)
Alcohols
Carbamic acid esters
Organophosphates
1- Edrophonium
1- Neostigmine
2-Pyridostigmine
2- Physostigmine (eserine)
1- Echothiophate
2-Isoflurophate,
3-Soman,
4-Parathion,
5- Malathion
6- Dyflos
The action of the drug is
very brief. It is used mainly
for diagnostic purposes
(myasthenia gravis )
Neostigmine is not absorbed
and does not enter CNS
Physostigmine is absorbed from
all sites including conjunctiva
and enters CNS
All
organophosphorous
compounds
are
well
absorbed from all sites of
administration and enter
the
CNS
except
echothiophate
Most of these compounds
developed as war gases and
pesticides as well as for
clinical use.
Reversible inhibition after Reversible inhibition after 30
2-10 min (short-acting
min to 6 hours (intermediateanticholinesterases)
acting anticholinesterases)
Irreversible inhibition
(can be reversed by
pralidoxime)
Cholinesterase inhibitors (Cont’d)
Pharmacological effects:
CNS
Tertiary compounds, such as physostigmine, and the non-polar
organophosphates penetrate BBB and affect the brain
Low concentrations cause alertness. High concentrations cause initial
stimulation and convulsions followed by depression coma.
Autonomic
cholinergic
synapses:
(Eye, GIT,
Bronchioles, and
urinary bladder
Cardiovascular
system,
glands…etc)
Increased secretions from salivary, lacrimal, bronchial and
gastrointestinal
glands;
increased
peristaltic
activity;
bronchoconstriction; bradycardia and hypotension; pupillary
constriction; fixation of accommodation for near vision; fall in
intraocular pressure.
Acute anticholinesterase poisoning causes severe bradycardia,
hypotension and difficulty in breathing
Neostigmine and pyridostigmine tend to affect neuromuscular
transmission more than the autonomic system, whereas physostigmine
and organophosphates show the reverse pattern.
Neuromuscular
junction
Therapeutic doses increases strengh of contraction
In large doses, such as can occur in poisoning, anticholinesterases
initially cause muscle twitching and fibrilation. Later, paralysis may
occur due to depolarisation block, which is associated with the buildup of ACh in the plasma and tissue fluids.
Cholinesterase inhibitors (Cont’d)
Therapeutic uses:
Disease
Mechanism of action
Drug
1- Glaucoma
Contraction of the ciliary muscle and circular Physostigmine
sphinctor muscle of the iris that increasing the echothiophate
outflow of the aqueous humor
(as eye drops)
2- Postoperative
To reverse the action of non-depolarising
neostigmine
neuromuscular-blocking drugs.
3- Urinary retention
Non-obstructive urine retention
4- Myasthenia gravis
a-treatment
neostigmine
neostigmine,
pyridostigmine
b-test for
myasthenia gravis
and to distinguish weakness caused by edrophonium
anticholinesterase overdosage ('cholinergic
crisis') from the weakness of myasthenia itself
('myasthenic crisis'):
5- Dementia like
Alzheimer's dieases
cholinesterase inhibitors may act to reduce
neurotoxicity by inhibiting formation of Aβ, and
therefore the progression of AD as well as
producing symptomatic benefit
donepezil,
tacrine
rivastigmine
and
galantamine
Cholinesterase inhibitors (Cont’d)
Toxicity:
Acute toxicity (cholinergic crisis):
Treated by atropine and pralidoxime
A- miosis, nausea, vomiting, diarrhea, salivation, sweating,
lacrimation cutaneous vasodilation, and bronchial
constriction and excessive urination
B- These manifestations are followed by:
central stimulation, which cause convulsions and may
progress to coma and respiratory arrest;
skeletal muscle paralysis
hypertension and cardiac arrhythmias.
Ganglion Stimulants
• Autonomic ganglia (both sympathetic and
parasympathetic)
and
neuromuscular
junctions contain nAChRs (Nn and Nm
respictively). Most nAChR agonists affect both
ganglionic and neuromuscular junction
receptors.
• Nicotine
(at
low
conc),
lobeline,
Tetramethylammonium
(TMA)
and
dimethylphenylpiperazinium (DMPP) affect
ganglionic receptors preferentially.
Ganglion Stimulants (cont.)
• Nicotine and lobeline are tertiary amines
found in the leaves of tobacco and lobelia
plants
• Nicotine is the most commonly
encountered nicotinic agonist
• It has Biphasic action on ganglionic nAChR
• Stimulates at low doses
• Stimulates then blocks at high doses
• Nicotine works in both PNS and CNS .
• One of the most toxic effects is the
dependence-producing
psychoactive
compounds overall
Ganglion Stimulants (cont.)
Pharmacological actions of nicotine:
Nicotine has a complex effect in autonomic ganglia
A- At low dosages it stimulates ganglionic nAChRs (
causing marked activation and initiation of action
potentials in postganglionic neurons) thus enhancing
both sympathetic and parasympathetic
neurotransmission
The initial response therefore often resembles
simultaneous discharge of both the parasympathetic and
the sympathetic nervous systems
- Regarding CVS, the effects of nicotine are chiefly
sympathomimetics (increased HR, force of contraction
and vasoconstriction)
Ganglion Stimulants (cont.)
- In the GI, glands and urinary tracts, the effects are
largely parasympathomimetic (Increased tone,
motility and secretions of the GIT, increased
bronchial, salivary and sweat secretions, and also
urinary outflow).
B- As nicotine dosages increase, nicotine possesses some
antagonist effect at nicotinic receptors.
Prolonged exposure results in depolarizing blockade of the
ganglia (initial increase then decrease in HR, vasodilation
)
Ganglion Stimulants (cont.)
• Nicotine have the ability to cross the BBB and
affects CNS (especially brainstem and cortex)
• It cause initial stimulation followed by
depression upon increasing the dose.
• Nicotine may induce tremor, vomting, and
stimulation of the respiratory center. At still
higher levels, nicotine causes convulsions, which
may terminate in fatal coma
• Nicotine is one of the most dependenceproducing drugs.
Ganglion Stimulants (cont.)
• Most of ganglion stimulants are not used
clinically, but only as experimental tools.
• Only nicotine is used clinically in the
form of transdermal patches, gums, SL
tablets which is used as an aid to smoking
cessation.
Anticholinergic drugs
Muscarinic blockers
Non-selective
Selective
Nicotinic
blockers
Ganglionic
blockers
Neuromuscular
blockers
1- Muscarinic blockers
Parasympatholytics
• Muscarinic receptor antagonists
• Muscarinic receptor antagonists
are competitive
antagonists whose chemical structures usually contain
ester and basic groups in the same relationship as ACh.
• Two
main subgroups of muscarinic antagonists ar
erecognized:
•
1Naturally
occurring
(non-selective)
compounds: most of these compounds are alkaloids
found in solanaceous plants like Atropa belladonna and
Datura stramonium, e.g. atropine, hyoscine
(scopolamine).
•
2- Synthetic (more selective) derivatives of
atropine: like Ipratropium (broncheal muscles),
Cyclopentolate (eye), Oxybutynin (urinary bladder) ,
and Pirenzepine (M1-selective).
1-Atropine
• Atropine is an alkaloid derived from the plant
• Atropa belladonna.
• It is act as non selective competitive inhibitor of Ach
on muscarinic receptors both peripherally and
centrally.
Pharmacokinetics:
• It is a tertiary ammonium, lipid-soluble compound
that is readily absorbed from the GIT or conjunctival
sac and cross BBB.
• Metabolized in the liver, excreted in urine.
• It Has short duration of action on most organs
except eye
Atropine (cont.)
Pharmacological effects:
Effects on CNS
•
•
•
•
Atropine produces mainly excitatory effects on the CNS.
At low doses, this causes mild restlessness.
higher doses cause agitation and disorientation.
In atropine poisoning, marked excitement , irritability,
hyperactivity and a hyperthermia. These central effects are
the result of blocking mAChRs in the brain, and they are
opposed
by
anticholinesterase
drugs
such
as
physostigmine, which is an effective antidote to atropine
poisoning.
Atropine pharmacological effects (cont.)
•
Atropine also affect the extrapyramidal system,
reducing the involuntary movement and rigidity of
patients
with
Parkinson's
disease
and
counteracting the extrapyramidal side effects of
many antipsychotic drugs.
Atropine pharmacological effects (cont.)
Effects on cardiovascular system
A- Heart:
- Initial bradycardia (central) followed by
tachycardia ( peripheral M2 blockade ).
- ↑ AV conduction ( + ve chronotropic effect ).
B- Blood vessels:
- Most of resistance vessels have no cholinergic innervation
(arterial blood pressure is unaffected).
- ↓ Vasodilatation induced by cholinomimetics.
At relatively high dose, atropine produces cutaneous
vasodilatation (atropine flush).
Atropine pharmacological effects (cont.)
Effects on gastrointestinal tract
- Relaxation of smooth muscles (constipation).
- ↓ GIT tone and motility → Antispasmodic
effect. This requires larger doses of atropine.
- ↑ Sphincter contractions.
-↓ Gastric secretion
Atropine pharmacological effects (cont.)
Effects on secretions
– ↓ Salivary secretion → ( Dry mouth ).
– ↓ Sweating → Dry skin → Fever in infants
–
and children.
– ↓Bronchial secretion → ↑ Viscosity. Mucociliary
clearance in the bronchi is inhibited, so that
residual secretions tend to accumulate in the
lungs
– ↓ Lacrimal secretion → Sandy eye.
Atropine pharmacological effects (cont.)
Effects on the eye
‐ The pupil is dilated (Passive mydriasis) ,due to paralysis
of circular muscle
‐ Cycloplegia (loss of accommodation for near vision) ,
due to paralysis of ciliary muscle.
‐ Loss of light reflex (eye pupil becomes unresponsive to
light).
‐ It increase IOP, this is unimportant in normal
individuals, it can be dangerous in patients suffering
from closed-angle glaucoma. .
Atropine pharmacological effects (cont.)
Effects on other smooth muscle
• Bronchial smooth muscles are relaxed by atropine
(broncheodilation) and bronchial secretions are
decreased.
• Reflex bronchoconstriction (e.g. during anaesthesia) is
prevented by atropine (preanaestetic medication).
• Biliary smooth muscles are relaxed by atropine
(management of bilary colic)
• Urinary tract smooth muscles are relaxed by atropine
- Relaxation of the ureter and bladder smooth
muscles
- Contraction of sphincter (urinary retention)
Atropine (cont.)
Clinical uses:
1. preanesthetic medication to :
-↓ salivary & bronchial secretion and inhibit
reflex bronchoconstriction
- Protect the heart from excessive vagal tone.
2. Antispasmodic in renal , bilary, and intestinal
colics.
3. It is also useful in the treatment of peptic ulcer
(decrease gastric secretions).
4.
antidote
in
cholinomimetic
or
organophosphorous poisoning.
5. Treatment of sinus bradycardia after myocardial
infarction(to prevent vagal discharge).
6. Ophthalmic administration is used for producing
mydriasis. This helps in fundus examination
Atropine (cont.)
Adverse effects
•
•
•
•
•
•
Blurred vision
Tachycardia and rapid pulse
Urinary
retention
(especially
in
eldry)Constipation.
Dryness of mouth , Sandy eye
Hyperthermia ,especially in children, and Atropine
flush.
Hallucination, excitationan, restlessness, confusion
and disorientation (Toxic dose). Physostigmine is
the antidote in case of atropine poisoning.
2- Hyoscine (Scoplamine)
Pharmacological effects:
• Scoplamine Like atropine,
possesses strong
antimuscarinic actions.
• It is more potent than atropine in producing mydriasis
, cycloplegia, and a decrease in bronchial, salivary, and
sweat gland secretions.
• It is less potent than atropine in its cardiac, bronchial
muscles, and intestinal effects.
• Atropine has a longer duration of action, than
scopolamine.
2- Hyoscine (Scoplamine)
• In therapeutic doses , it causes marked CNS
depression and sedation, but has similar effects to
atropine in high toxic doses.
• It also has a useful antiemetic effect (CTZ) and is used
in treating motion sickness.
Therapeutic uses:
• Preanesthetic medication
• Antiemetic action (Motion sickness) by oral orTD
patchs.
• To facilitate endoscopy and GIT radiology by relaxing
GIT smooth muscle (spasmolytic)
Side effects:
• Similar to those of atropine.
3- Synthetic atropine dreivatives
These drug are more selective than atropine and mostly
have minimal effects on CNS. They are classified
according to tissue selectivity and clinical uses.
M1 Muscarinic Receptor Antagonists:
e.g. Pirenzepine and Telenzepine. They are useful for
treatment of peptic ulcer
M3 muscarinic Receptor Antagonists:
e.g. Oxybutynin, tolterodine and darifenacin . They
are new drugs that act on the bladder to inhibit
micturition, and are used for treating urinary
incontinence and urinary colics.
3- Synthetic atropine dreivatives
Antimuscarinic drugs for ophthalmic applications:
e.g. Cyclopentolate and Tropicamide. They mostly
used to dilate the eye pupil for funduscopic examination
of the eye. They shorter duration of action than atropine
(12hrs, 6 hrs and 5-7 days).
Antimuscarinic drugs for antispasmodic effects:
e.g. Dicyclomine, Oxyphencyclimine, Propantheline,
Glycopyrrolate
,
and
Hyoscine-butylbromide
(Buscopan) . They are useful for spasms of the GIT, bilary
duct, ureters, especially in those severe conditions as an
irritable bowel syndrome, billary or uretheral stones
3- Synthetic atropine dreivatives
Antimuscarinic drugs for treatment of neurological
disorders :
e.g. Benzhexol, Benztropine. They are mostly used in
management of movement disorders associated with
Parkinson’s disease and extrapyramidal side effects of
antipsychotic drugs.
Antimuscarinic drugs for treatment of respiratory
disorders :
e.g. Ipratropium and Tiotropium. They are mostly
used in treatment of asthma and COPD. Taken by
inhalation as aerosol to produce bronchodilation and
decrease bronchial secretions.
2- Nicotinic blockers
AGanglion
blockers
Compounds like
•
•
- Quaternary ammonium compounds
e.g. Hexamethonium,
tetraethylammonium, tubocurarine
•
- Amines (secondary/tertiary)
•
e.g. Mecamylamine, Pempidine
•
- Monosulfonium compound
•
e.g. Trimethaphan
• - and nicotine (at high concentration)
have the ability to block the autonomic
ganglia.
• Ganglionic blockers reduce transmission
in
all
autonomic
ganglia,
both
sympathetic and parasympathetic
Ganglion blockers (cont.)
Pharmacological effects:
• In some sites, sympathetic activation seems to
predominate over parasympathetic, while in other
sites, the opposite is true
• Ganglionic blockade thus "covers" the predominant
system
Effects
of Ganglionic
Blockade
ORGAN
DOMINANT
TONE on Organ
EffectSystems
of ganglionic
blockade
HEART
Para-sympathetic
Tachycardia (palpitation)
BLOOD VESSELS
Sympathetic
Dilatation, abolition of
reflexes, syncope, hypotension
IRIS
Para-sympathetic
Mydriasis (photophobia)
CILIARY MUSCLES
Para-sympathetic
Cycloplegia (blurring of near
vision)
INTESTINES
Para-sympathetic
Hypomotility (constipation)
BLADDER
Para-sympathetic
↓ tone (difficulty in
micturition)
MALE SEXUAL FUNCTION Para-sympathetic
Inhibition of erection
And ejaculation (Impotence)
SALIVARY GLANDS
Para-sympathetic
Inhibition of watery salivation
(dry mouth or xerostormia)
SWEAT GLANDS
Sympathetic (cholinergic)
Inhibition of sweating
(anhydrosis)
Ganglion blockers (cont.)
• In practice, the CVS effects are the most important ones:
- A marked fall in arterial blood pressure results mainly from
arteriolar vasodilatation.
- Most cardiovascular reflexes are blocked. In particular, the
venoconstriction, which occurs normally when a subject stands
up, and which is necessary to prevent the central venous pressure
from falling sharply, is reduced. Standing thus causes a sudden
fall in cardiac output and arterial pressure (postural
hypotension) that can cause fainting or even shocking.
- Similarly, the vasodilatation of skeletal muscle during exercise is
normally accompanied by vasoconstriction elsewhere (e.g.
splanchnic area) produced by sympathetic activity. If this
adjustment is prevented, the overall peripheral resistance falls
and the blood pressure also falls (postexercise hypotension).
Ganglion blockers (cont.)
Therapeutic Uses:
Use of the ganglion blockers is infrequent due
to many severe side effects accompanied with.
Mecamylamine is being studied for
possible use in reducing nicotine craving in
patients attempting to quit smoking.
Ganglion blockers (cont.)
Trimethaphan
is a very short- acting
drug that can be administered as an IV infusion
in certain anaesthetic procedure to control
blood pressure during anaesthesia.
Also, used to minimize bleeding during certain
kinds of surgery.
Trimetaphan can also be used in the treatment
of hypertensive emergencies and during
electroconvulsive therapy.
Ganglion blockers (cont.)
Side effects
•Orthostatic hypotension
•Blurred vision
•Urinary retention, constipation
•Sexual impotence
B- Neuromuscular blockers
• Neuromuscular junction (NMJ) is the junction of
the nerve terminal of a motor neuron with the motor
end plate (skeletal muscle fiber).
• Skeletal muscle relaxants: are groups of drugs which
affects skeletal muscle function and decreases the
muscular tone. It includes two categories of drugs,
spasmolytics and neuromuscular blockers.
Skeletal muscle
relaxants
Spasmolytics
(Centerally-acting
sk. Muscle
relaxants)
Neuromuscular
blockers
(Peripherally-acting
sk. Muscle relaxants)
Neuromuscular blockers (cont.)
•
Spasmolytics
are a group of drugs was traditionally
know as “centrally-acting skeletal muscle relaxants”. However, at
least one of these agents (dantrolene) has no significant central
effects.
•
Spasmolytic drugs are used in the treatment of muscle spasm
and immobility associated with strains, sprains, and injuries of
the extremities, back and neck. In addition to their usage to
alleviate painful muscular spasm
associated with many
neuropathological disorders.
• They have a diverse mechanisms of action, but they are not
directly affect transmission within motor end plates.
• Guaifenesin , Chlordiazepoxide ,Baclofen , Chlorphenesin,
and Dantrolene are examples of spasmolytics
Neuromuscular blockers (cont.)
• Neuromuscular blockers are group of drugs
act peripherally post-synaptically on motor end plate
to interfere with transmission at muscular nicotinic
receptors (NmAChRs). They lack any CNS effects and
may share some charecterestics with autonomic
ganglionic blockers.
• They are used mainly to produce a certain level of
muscle paralysis for patients requiring ventilatory
assistance during surgical procedures and in intensive
care units.
• Two different kinds of functional blockade may occur
at the neuromuscular endplate, and hence clinically
used drugs fall into two categories
Competitive neuromuscular blockers
These group of drugs act as
competitive antagonists with Ach at
the site of NmAChRs.
No depolarization of postjunctional
membrane.
Cholinesterase
inhibitors
(like
neostigmine) can reverse
this
blockade.
Examples:
d-tubocurarine,
Gallamine,
Atracurium,
Pancuronium, Vecuronium, and
Mivacurium
Ach
T
Nicotinic receptor
NMJ
Ion
channel
Competitive neuromuscular blockers (cont.)
Pharmacokinetic aspects :
• Competitive neuromuscular-blocking agents are used
mainly in anaesthesia to produce muscle relaxation. They
are given intravenously (inactive when used orally)
• Most of the non-depolarising blocking agents are
metabolised by the liver or excreted unchanged in the
urine. With
exceptions being atracurium, and
mivacurium, which are hydrolysed by plasma
pseudocholinesterase.
• Their duration of action varies between about 15 minutes
and >2 hours , by which time the patient regains enough
strength to cough and breathe properly, although residual
weakness may persist for much longer.
Competitive neuromuscular blockers (cont.)
Pharmacological actions:
• Skeletal
muscle
relaxation
is
the
main
pharmacological effect. This effect is mainly due to
motor paralysis. The first group of muscles to be
affected are the extrinsic eye muscles (causing double
vision) and the small muscles of the face, limbs and
pharynx area (causing difficulty in swallowing).
• Respiratory muscles are the last to be affected and the
first to recover.
Competitive neuromuscular blockers (cont.)
Unwanted side effects:
1- Hypotension is the main side effect many competitive
NMBs (d-tubocurarine, atracurium and mivacurium),
this happened mainly due to ganglion block effect (dtubocurarine).
2- Also, stimulation of histamine release from mast cells
which can help in reduction of arterial BP and also give rise
to bronchospasm in sensitive individuals. Gallamine and
pancuronium lack these side effects.
3- Gallamine, and pancuronium, block mAChRs,
particularly in the heart, which results in tachycardia.
Anticholinesterase drugs (e.g. neostigmine) are very
effective in overcoming the blocking action of competitive
agents.
Competitive neuromuscular blockers (cont.)
d-Tubocurarine (curare):
It is a plant alkaloid that has slow
onset of action (> 5 min) and
longer duration(1-2 h). It also
affect autonomic aganglia.
The main side effects is
Bronchoconstriction
and
hypotension. In addition to
other side effects related to its
ganglion blocking activity
(
blurred vision , urine retention ,
conistipation
and
male
impotence)
D-tubocurarine
Competitive neuromuscular blockers (cont.)
Gallamine (Flaxedil):
It is synthetic compound has less potent
NM blocking activity than curare ( 1/5
potency)
It has shorter onset (2-3 min) and longer
duration ( > 2h) than d-tubocurarine.
It is execreted unchanged mainly by
kidney. It is contraindicated in renal
failure
Main side effect is “tachycardia” due to
an atropine-like action and stimulation
of NA release from adrenergic nerve
endings.
Gallamine
Competitive neuromuscular blockers (cont.)
Atracurium:
It has similar potentency as curare (1-1.5 times) with intermediate
onset (2-3 m) and intermediate duration (< 30 min)
It has unusual mechanism of elimination (spontaneous nonenzymaic hydrolysis in plasma at body pH); degradation slowed by
acidosis.
It is widely used especially in liver failure & kidney failure
The main side effects is the transient hypotension (due to
histamine release), but has no effect on muscarinic receptor nor
ganglia.
Competitive neuromuscular blockers (cont.)
Mivacurium:
It is new drug that is chemically-related to atracurium.
It has Fast onset (∼2 min) and short duration (∼15 min).
It is metabolized by plasma pseudocholinesterases
(Longer duration in patient with liver disease or genetic
cholinesterase deficiency).
Transient hypotension is the main side effect.
Competitive neuromuscular blockers (cont.)
Pancuronium:
It is the first steroid-based
compound that is more potent than
curare ( 6 times ). It has Intermediate
onset (2-3 min)
and slight long
duration (>2h)
Excreted mainly by the kidney
(
80 % ).
Tachycardia is the main side effect
(due to an atropine-like action and
stimulation of NA release from
adrenergic nerve endings).
Pancuronium
Competitive neuromuscular blockers (cont.)
Vecuronium:
It is more potent NMBs than curare (6times) with
Intermediate onset (2-3 min) and Intermediate
duration (30-40 min)
It is metabolized mainly by liver. Its metabolites have
some activity. It has few side effects (no histamine
release, no ganglion block and no antimuscarinic
action). Occasionally causes prolonged paralysis,
probably owing to active metabolite
It is widely used.
Depolarizing neuromuscular blockers
This group of NMBs have the ability to combine with
NmAChRs and stimulate motor end plates by initiation of
membrane depolarization This initial depolarization is
accompanied by transient twitching of the skeletal muscle
(fasciculation) Phase I (phase of initial
depolarization).
Phase I block is augmented not reversed by
anticholinestrases.
Depolarizing neuromuscular blockers (cont.)
• Continuous exposure to depolarizing NMBs (not liable to
be hydroilized with cholinesterase) and persistent
depolarization, the skeletal muscle tone cannot be
maintained, decreases and the membrane become
gradually repolarized (as the sodium channel closes),
and the membrane cannot be depolarized by Ach as
long as the NMB is present , therefore, this continuous a
functional muscle fatigue occurs and paralysis (flaccid
paralysis; muscles are weak and have little or no tone)
leads to depolarization Phase II ( Phase of
desensitization block of the membrane) .
This phase reversed by anticholinesterase.
Succinylcholine and decamethonium are examples of this class
of drugs.
Phase I (Depolarization)
Phase II (Desensitization block)
Depolarizing neuromuscular blockers (cont.)
Succinylcholine (suxamethonium):
It has a short onset of action ( 1 min. ) and short duration
of action (5-10 min.). It must be given by continuous IV
infusion if prolonged paralysis is required. It is destroyed
by pseudocholinesterase.
• Mostly used for brief procedures (e.g. tracheal intubation,
electroconvulsive
shock
therapy).
•
Succinylcholine
Succinylcholine (cont.)
Side effects:
• Bradycardia ( due to muscarinic agonist effect) .this could
be prevented by atropine.
• Cardiac dysrhythmias or even cardiac arrest (increase K+
permeability of the motor endplates causes a net loss of K+
from muscle and increased plasma K+ concentration
“hyperkalemia”). It should be avoided in patients with
burns or severe trauma .
• Raised intraocular pressure (nicotinic agonist effect on
extraocular muscles).
• Prolonged paralysis or succinylcholine apnea in patients
with liver insuffeciency or genetic deficiency of plasma
cholinesterase
• Increase the intragastric pressure and may leads to
regurgitation of gastric content to esophagus.
•
Succinylcholine (cont.)
Malignant hyperthermia:
• Malignant hyperthermia (MH) is a rare inherited
condition, due to a mutation of the Ca2+ release channel of
the sarcoplasmic reticulum (the ryanodine receptor
“RYR1”), which results in intense muscle spasm and a
dramatic rise in body temperature with an increased heart
rate and respiratory rate.
• This is a disorder that can be considered a geneenvironment interaction. In most persons with Malignant
hyperthermia susceptibility, they have few or no
symptoms unless they are exposed to a triggering agent.
The most common triggering agents are volatile anesthetic
gases (such as Halothane,and Isoflurane)
, the
depolarizing muscle relaxants (Suxamethonium and
Decamethonium) catecholamines (such as EP,NE and
DA), phenothiazines (such as Chlorpromazine, and
Promethazine), and MAO inhibitors (such as Phenelzine,
Moclobemide, and Selegiline).
Succinylcholine (cont.)
• Some other factors could trigger symptoms of MH in
suceptable individuals like physical exercise and hot
environment.
• The condition carries a very high mortality (about
65%) and is treated by IV administration of
Dantrolene, a drug that inhibits muscle contraction
by preventing Ca2+ release from the sarcoplasmic
reticulumin addition to discontinuation of
triggering agents, and supportive therapy to control
hyperthermia, and acodosis.