CH 3 Worksheet - introduction to the atomic structure
advertisement

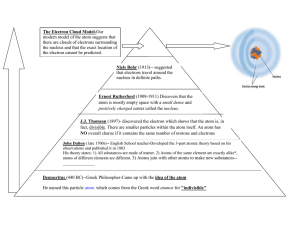
INTRODUCTION TO ATOMIC STRUCTURE 1. Until 1897, chemists accepted the theory that the atom was the smallest particle of matter obtainable. Atoms were believed to be indivisible P-~ Now we know that die atom is composed of at least three kinds of particles: __________ electrically positive; and electrically negative; electrically neutral. 2. (a) According to the modem picture of an atom, itconsists of a very dense central portion, called the _________________ , surrounded by that make up the volume of the atom but add little to its _ ( b) The nucleus consists of a number of and tightly bound together by a kind force called the .......................................... force. (c) Every atom of the same element has the same number of _. . in its nucleus, although the number of ........................................ may vary. (d) The number of electrons in the atom is equal to 3.Electrons in the atoms of different elements are (alike,different) 4.Protons in the atoms of different elements are (alike, different) 5.Neutrons in the atoms of different elements are (alike, different) 6.In what two respects do the atoms of different elements differ from each other? 7.(a) The mass of a proton is approximately (b) The mass of a neutron is approximately ________ (c) The mass of an electron is approximately atomic mass units amu. amu 8. Because of the small mass of the c:lectron, the mass of an atom is practically the same as the mass of its 9. The simplest and lightist of the atoms is an atom . consists of. and it has _________________ . Its nucleus ,- electron(s). 10.The mass of a hydrogen atom is approximately _________ a.m.u. 11.The atomic number Z of an atom is equal to .. ......... ......................................... ...... ............... ........................................................................ ......... ......................................... ...... ............... ........................................................................ ......... ......................................... ...... ............... ........................................................................ ......... ......................................... ...... ............... 12. The mass number A. of an atom is equal to .. 13. The mass number of an atom is therefore equal to the atomic (mass, number) the nearest integer. , rounded to