Chapter 4
advertisement

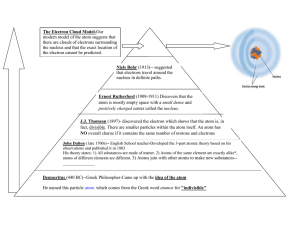
Atomic Structure Democritus Believed all matter consisted of extremely small particles that could not be divided Called these particles atoms, which means “uncut” or “indivisible” He thought there were different types of atoms with specific sets of properties ◦ Atoms in liquids were round and smooth ◦ Atoms in solids were rough and prickly Aristotle Didn’t think there was a limit to the number of times matter could be divided Thought all substances were made from only four elements ◦ Earth ◦ Air ◦ Fire ◦ Water Dalton’s Atomic Theory: All matter is made up of individual particles called atoms, which cannot be divided All elements are composed of atoms All atoms of the same element have the same mass, and atoms of different elements have different masses Compounds contain atoms of more than one element In particular compounds, atoms of different elements always combine in the same way J.J.Thomson Use an electric current to learn more about atoms Discovered atoms contain negatively charged particles, or electrons All atoms are neutral The atom is filled with a positively charged mass of matter that has negative charges (electrons) evenly scattered throughout it. Ernest Rutherford Gold Foil Experiment Deflected particle Undeflected particle Alpha particles Gold atoms Slit Screen Alpha particles Source of alpha particles Nucleus Ernest Rutherford The positive charge of an atom is not evenly spread throughout the atom. Positive charge is concentrated in a very small, central area. The nucleus of the atom is a dense, positively charged mass located in the center of the atom. The Houston Astrodome occupies more than nine acres and seats 60,000 people. If the stadium were a model for an atom, a marble could represent its nucleus. The total volume of an atom is about a trillion (1012) times the volume of its nucleus. Assessment Questions 1. Dalton’s theory did not include which of the following points? a. All elements are composed of atoms. b. Most of an atom’s mass is in its nucleus. c. Compounds contain atoms of more than one element. d. In a specific compound, atoms of different elements always combine in the same way. Assessment Questions 1. Dalton’s theory did not include which of the following points? a. All elements are composed of atoms. b. Most of an atom’s mass is in its nucleus. c. Compounds contain atoms of more than one element. d. In a specific compound, atoms of different elements always combine in the same way. ANS: B Assessment Questions 2. J. J. Thomson’s experiments provided the first evidence of a. b. c. d. atoms. a nucleus. subatomic particles. elements. Assessment Questions 2. J. J. Thomson’s experiments provided the first evidence of a. b. c. d. atoms. a nucleus. subatomic particles. elements. ANS: C Assessment Questions 3. The concept of an atom as a small particle of matter that cannot be divided was proposed by the ancient Greek philosopher, Democritus. True False Assessment Questions 3. The concept of an atom as a small particle of matter that cannot be divided was proposed by the ancient Greek philosopher, Democritus. True False ANS: T A proton is a subatomic particle with a charge of +1 that is found in the nucleus of an atom. Every nucleus must contain at least one proton. A neutron is a neutral (has no charge) subatomic particle that is found in the nucleus of an atom. It has a the same mass as a proton. An electron is a subatomic particle with a charge of -1 that is found outside the nucleus. An electron has no mass. The atomic number of an element is the number of protons in an atom of that element. Every element has its own atomic number. If the atomic number of two elements is the same, they are the same element. The mass number of an atom is the sum of the protons and neutrons in the nucleus of that atom. Every atom of the same element has the same number of protons and electrons. Every atom of the same element does not have the same number of neutrons. Isotopes are atoms of the same element that have different numbers of neutrons and different mass numbers. To distinguish one isotope from another, the isotopes are referred by their mass numbers. Oxygen has 3 isotopes ◦ oxygen-16 ◦ oxygen-17 ◦ oxygen-18 Hydrogen has 3 isotopes ◦ hydrogen-1 ◦ hydrogen-2 ◦ hydrogen-3 Assessment Questions 1. In which way do isotopes of an element differ? a. b. c. d. number of electrons in the atom number of protons in the atom number of neutrons in the atom net charge of the atom Assessment Questions 1. In which way do isotopes of an element differ? a. b. c. d. number of electrons in the atom number of protons in the atom number of neutrons in the atom net charge of the atom ANS: C Assessment Questions 2. Of the three subatomic particles that form the atom, the one with the smallest mass is the neutron. True False Assessment Questions 2. Of the three subatomic particles that form the atom, the one with the smallest mass is the neutron. True False ANS: F, electron Bohr’s Model of the Atom Electrons move with constant speed in fixed orbits around the nucleus, like planets around a sun. Each electron in an atom has a specific amount of energy. Energy Levels When an atom gains or loses energy, the energy of an electron can change. ◦ ◦ The possible energies that electrons in an atom can have are called energy levels. An electron cannot exist between energy levels. An electron in an atom can move from one energy level to another when the atom gains or loses energy. Electron Electrons gain or lose energy when they move between fixed energy levels Nucleus Bohr Model The movement of electrons between energy levels explains the light you see when fireworks explode. Electrons move to higher energy levels when they absorb energy When those electrons move back to lower energy levels, they release energy. Some of that energy is released as visible light. Different elements emit different colors of light because no two elements have the same set of energy levels. Bohr’s model was improved as scientists made further discoveries. Bohr correctly assigned energy levels to electrons, but electrons do not move like planets in a solar system. Today, scientists use probability when trying to predict the locations and motions of electrons in atoms. An electron cloud is a visual model of the most likely locations for electrons in an atom. The electron cloud is a visual model of the probable locations of electrons in an atom. The probability of finding an electron is higher in the denser regions of the cloud. The nucleus contains protons and neutrons Electron Cloud Model The electron cloud represents all the orbitals in an atom. An orbital is a region of space around the nucleus where an electron is likely to be found. An electron configuration is the arrangement of electrons in the orbitals of an atom. When all the electrons in an atom have the lowest possible energies, the atom is said to be in its ground state. The most stable electron configuration is the one in which the electrons are in orbitals with the lowest possible energies. The ground state of a person is on the floor. A gymnast on a balance beam is like an atom in an excited state—not very stable. When she dismounts, the gymnast will return to a lower, more stable energy level. Assessment Questions 1. According to Bohr’s model of the atom, which of the following can happen when an atom gains energy? a. b. c. d. An atom returns to its ground state. A neutron can be changed into a proton. A proton can move to a higher energy level. An electron can move to a higher energy level. Assessment Questions 1. According to Bohr’s model of the atom, which of the following can happen when an atom gains energy? a. b. c. d. An atom returns to its ground state. A neutron can be changed into a proton. A proton can move to a higher energy level. An electron can move to a higher energy level. ANS: D Assessment Questions 2. How does the modern atomic theory describe the location of electrons in an atom? a. Electrons move randomly in space around the nucleus. b. Electrons can be described as a cloud based on probable locations. c. Electrons orbit the nucleus in the same way that planets orbit the sun. d. Electrons move in a spiral pattern if increasing distance from the nucleus. Assessment Questions 2. How does the modern atomic theory describe the location of electrons in an atom? a. Electrons move randomly in space around the nucleus. b. Electrons can be described as a cloud based on probable locations. c. Electrons orbit the nucleus in the same way that planets orbit the sun. d. Electrons move in a spiral pattern if increasing distance from the nucleus. ANS: B Assessment Questions 3. What is meant when an atom is said to be in its ground state? a. There is no net charge on the atom. b. The number of protons equals the number of neutrons. c. The atom’s electrons all have the lowest possible energies. d. It is the isotope with the least number of neutrons. Assessment Questions 3. What is meant when an atom is said to be in its ground state? a. There is no net charge on the atom. b. The number of protons equals the number of neutrons. c. The atom’s electrons all have the lowest possible energies. d. It is the isotope with the least number of neutrons. ANS: C