Iron pill analysis.13

Analysis of an Iron Supplement via Redox Titration
Chem. 319 Ms. Hobbie
In this lab you will act as an FDA (Food and Drug Administration) chemist and check to see if the labeling of an iron supplement is correct. A bottle of iron(II) sulfate (ferrous sulfate) pills was purchased at Walgreen’s. You will carry out a redox titration in order to determine the mass of iron in the pills. You can then compare your value to that listed on the bottle (65 mg per pill).
You'll need to dissolve the pills in a dilute sulfuric acid solution and then titrate with a good oxidizing agent: a solution of potassium permanganate (KMnO because the MnO
4-
4
). The permanganate will cause the iron(II) ion to be oxidized. The permanganate ion is commonly used in redox titration reactions
is purple while the reduced species is colorless.
The end point of the titration can be difficult to observe because the oxidized iron species is quite yellow. In order to remove the yellow color and thus make the end point markedly sharper, phosphoric acid is added during the titration. The phosphoric acid forms an essentially colorless complex ion with the oxidized iron and allows for a better observation of the end point.
Procedure
Safety notes:
Permanganate ion is a very strong oxidizing agent. It will stain clothes and can react violently with paper or clothes (oxidizing them…) if large quantities are spilled and not cleaned up immediately.
Phosphoric acid is also caustic… handle with care and clean up any spills immediately.
NOTE: You will perform this procedure twice: the first time to get a ball-park estimate of the amount of titrant needed, the second time to be as accurate as you can in this analysis.
Dissolve iron pills in acid solution:
1.
2.
Grind up two iron pills using a mortar and pestle. Using a “rubber policeman” carefully scrape all
3.
Turn on your hotplate to the highest setting. the powder into a 125 mL Erlenmeyer flask.
Add about 75 mL of 0.5 M sulfuric acid solution to the flask and swirl the flask for a minute. You
4.
may notice some un-dissolved stuff (starch fillers in the pill). Don’t worry about that.
Drop in your stir bar and place the flask on the hot plate. An increased temperature will help dissolve the iron(II) in the pill and will also cause the titration reaction to occur at a faster rate.
Prepare your buret
Make sure a waste beaker is below the buret before you begin to fill it, and keep it there until you are ready to perform the titration.
5.
Fill the buret about 1/5 full with KMnO
4
(aq), rinse most but not all of this through the tip, and then fill the buret with the solution. Check that there are no air bubbles in the tip.
6.
Record the initial reading of the buret.
Perform the titration
7.
When the iron pill solution has reached a minimum temperature of 70 °C, remove the flask from the hot plate. Place under the buret, start the solution stirring and begin the titration.
8.
When a light yellow color develops in the iron solution during the titration, add 3 mL of 85% H
4
.
9.
After you reach a light pink endpoint, wait 1 minute to see if the color fades (this will indicate that there is still FeSO
4
(s) that is dissolving and reacting with the KMnO
4
(aq)).
3
PO
If the pink color fades, add KMnO
10.
Record a final reading of the buret and determine the volume of KMnO
Perform the titration a second time.
4
(aq) solution drop wise until you reach a stable pink endpoint.
4
(aq) added.
11.
Repeat the process, this time working to be as careful as possible.
12.
Clean-up directions are on the reverse of this sheet…
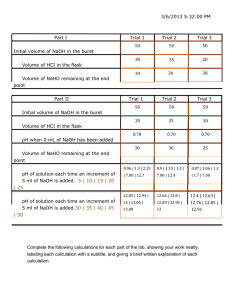
Data: Molarity of the KMnO
Ball-park trial:
4
(aq): _______________
Initial reading _______________ Final reading ______________
Careful Analysis: Initial reading ______________ Final reading ______________
Clean-up
Pour your reaction solutions and any leftover permanganate solution into your waste beaker
Rinse your buret three times: first with DI water, then with HCl, then with water again.
Dispose of your waste in the pink-labeled disposal container and rinse all glassware.
Analysis -- Present all this work cleanly and neatly…
1.
Write a balanced net ionic equation for the titration reaction used in this analysis: a.
Identify the oxidation and reduction products of the reaction. The last lab handout may be helpful in identifying the reaction products. Remember to OMIT spectator ions. b.
Write the two half reactions and balance them for both mass and charge. (AOHC) c.
Show how you combine these reactions to create a balanced net ionic equation for the reaction.
2.
Use your data and this balanced equation to determine the mass of iron present per pill in this supplement. Report your answer with the correct uncertainty (sig. figs.).
3.
Calculate the percent difference between your findings and the reported value of iron in each pill, 65 mg, also to the correct uncertainty.
Hand-in Guidelines:
Like a problem-set. Simply hand in the Analysis (above) and Additional Questions (below). These may be hand-written. Note that the Additional Questions should be your own work… no collaboration. On the labwork, as always, consider significant digits when you report your result(s).
Additional Questions – To be answered without the assistance of others.
4.
After the lab is over, the buret is cleaned of potassium permanganate solution by rinsing it with hydrochloric acid, which assists in the removal of any leftover purple color. a.
What is the “skeleton” reaction equation that occurs, allowing this to happen? (You may find your work on last week’s lab handout helpful here…) b.
Determine the net ionic equation for this reaction by using the method of balancing the two half reactions, AOHC. c.
Describe (one sentence) any safety precautions that should be taken into account when this reaction involves larger amounts of hydrochloric acid and permanganate than were involved in this clean-up procedure.
5.
While you were performing this lab, as a safety precaution I had solid sodium sulfite on hand. Had any large spill of permanganate occurred, I would have poured the solid on the spill, thus eliminating any danger of a violent redox reaction, and also to remove the color of the permanganate ion.
WITHOUT writing a balanced equation, explain in words why the sodium sulfite works… both to eliminate the danger of a violent oxidation reaction and to remove the color. Be specific: what are the products of the reaction?
--OVER--
6.
The chemistry technician prepared the KMnO
4
solution for this lab by first mixing the solution to an approximate molarity of 0.05 M. To standardize the solution, that is to determine the exact concentration of the solution with some confidence, the KMnO
4
was used in a titration with a known mass of oxalic acid dihydrate (H
2
C
2
O
4.
2H
2
O). The samples of oxalic acid dihydrate were weighed, dissolved in water and acidified with sulfuric acid. Each sample was titrated with the
KMnO
4
solution with the initial and final buret readings recorded. The following table is the data collected from the trials.
Mass Acid Initial Reading Final Reading
Trial #1 0.196 g 20.00 mL 32.30 mL
Trial #2
Trial #3
0.218 g
0.201 g
33.00 mL
27.15 mL
46.95 mL
40.15 mL a.
Write a balanced net ionic equation for the reaction between oxalic acid and permanganate ion in an acidic solution. Once again, the reactions performed on the last lab will be useful in b.
providing a skeleton ionic equation. Then AOHC.
For each trial, determine the exact molarity of the potassium permanganate solution. (Show c.
your work for one of these, then simply report the answers for the other calculations…)
Use the average to report a standardized value to the correct uncertainty, based on the data above.
7.
Here is a puzzle. A 1.637-g sample of solid vanadium(III) iodide is dissolved in a sulfuric acid solution. A redox titration is performed and equivalence is reached after 15.8 mL of 0.0400-M potassium dichromate is added. a.
Use the information recorded in the titration to determine the final oxidation state for vanadium. Helpful questions: a.
Is dichromate an oxidizing or reducing agent? What is its common product in acidic b.
b.
solution? What is the half reaction for this process?
How many moles of electrons are transferred to or from dichromate in this experiment?
Where did they come from/where do they go?
In preparing for this experiment, solid potassium dichromate is used to create the solution described above. Assuming the creation of a 100-mL sample of this 0.0400-M solution, explain how it was prepared. Be sure to provide specific measurements, describe any glassware used, and explain the order in which the chemist proceeded. (p. 188 is helpful here…)