Rachel bailey bio paper
advertisement

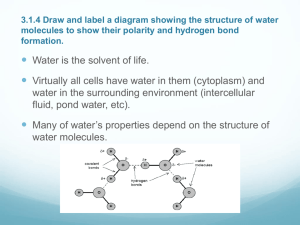
When it comes to water, everyone knows it is essential to life, but to what extent isn’t always clear. Water makes up 75% of Earth, and is what makes Earth able to sustain life. To understand how the water molecule is so useful and so unique, we must start by looking at it from a single molecule. A water molecule, or H2O, is formed by polar covalent bonds occurring between an oxygen molecule and two hydrogen molecules. What causes the water molecule to be polar is that the hydrogen ends have a positive charge and the oxygen end has a negative charge. Due to the polarity of the molecule, water can form hydrogen bond and have emergent properties that help make life possible here on earth. There are four emergent properties. We will be focusing on three, cohesion of the molecules, the way in which ice floats on water and the ability of using it as a solvent. The first property, cohesion, happens when water molecules become bonded by their opposing polar ends. Within the polar covalent bond of a water molecule there is a negative charge with the oxygen atom and a positive charge with the two hydrogen atoms. What happens next is that the two separate polar covalent bonded water molecules make a new type of bond with one another called a hydrogen bond. For instance, two water molecules will form a hydrogen bond by attracting one of the negative charged oxygen from one water molecule to the positive charge of the hydrogen on the other. Due to hydrogen bonds, water is a more structured liquid, and therefore it is useful in living organisms. Plants for example use the cohesion property of water molecules to move water from the ground, against gravity, up into the leaves and branches. What takes place here is that the water in the leaves evaporates, and in turn pulls on the water molecules further down. The hydrogen bonds pull on each other resisting gravity. Another emergent property of water is the way in which frozen water is less dense then liquid water, therefore ice floats on water. This property is significant due to the fact that if ice were to sink, then all bodies of water would eventually be solid ice and therefore be unable to sustain aquatic life or any life on earth. To better understand why ice is less dense than liquid water, we must look at the molecules as they solidify. It is important to know what shape a molecule becomes when it forms bonds with other molecules because the shape will determine its function. In the case of two or more water molecules forming hydrogen bonds the individual molecules are in the shape of a V with the positive charged hydrogen at the two tips. The positive hydrogen atom form one molecule will form a hydrogen bond with the negative charged oxygen atom at the bottom of the V from another water molecule. Those V shaped water molecules, in its liquid form are in a constant movement with one another reforming hydrogen bonds with the other water molecules. As the temperature drops and the water molecules slow down, the hydrogen bonds line up more even and repel each other at an even distance becoming a solid. The molecules also become further apart, thus making the solid form less dense then its liquid form making it possible to float. The last emergent properties is the use of water as a solvent. Water is not a universal solvent; however it is a versatile solvent that is able to dissolve many compounds. An example of this is the use of water to dissolve table salt (NaCl). Table salt is a compound of molecules that is composed of many ionic bonds between Sodium and Chloride, forming a crystalline structure. When the salt is added to the water, the water molecules are attracted to the sodium and chlorine atoms. One by one the wedge their way in and break of the atom, surrounding it with water molecules. This is due to the charged ends of the water molecule being attracted to the negative chlorine and the positive sodium atom. These properties are what contribute to the sustainability of life on Earth. As we can see there is a lot more to the use of water than we may have considered at first. Everyone knows we need water, but fail to realize the extent in which it affects our everyday lives. With the unique quality of water forming hydrogen bond we wouldn’t have some of these emergent properties. Without the emergent properties, trees would not be able to produce the oxygen we need to survive through the cohesion property. Polar bears would not have terrain to live and hunt if ice didn’t float. If water was not a great solvent that it is then we wouldn’t be able to digest foods. Which are all important in sustaining life on earth. With our individual papers we noticed a theme of not explaining the chemical properties and reactions that where taking place when we were writing about a major concept. We made corrections by going through and adding more specifics to the concepts, which in turn helped us to father understand the concepts at its chemical level. Water: Essential to Life Biology 1610-001 Dr. Sperry Assignment #1 Rachel Bailey Zach Coon Valerie Hempel Korry Wagstaff McCall