The Material World
advertisement

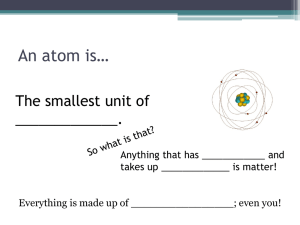
What is Matter??? Matter is anything that has mass, and takes up space examples: bowling balls have lots of mass - they are heavy - so they are made of matter you have mass, and you take up space, so you are made of matter air has only a little mass, but it has some, and takes up lots of space, so air is matter. any kind of material, solid, liquid, or gas is made of matter Matter (of any kind) is made up of extremely tiny particles. These particles are constantly in motion. At higher temperatures, these particles move faster. They would only stop moving at -273 degrees C. These particles are held together by forces of attraction, but never actually touch each other. Solids Liquids solids have definite shape and definite volume the particles in solids are held strongly in position, usually in some geometrical pattern the particles in a solid only move by vibrating - they can't leave their positions liquids have definite volume, but take the shape of their container the particles in a liquid are held closely together, but are free to move around the particles have enough energy so that they are not locked in position Gases gases have no definite shape or volume. They will spread out to fill any container that they are in. the particles of a gas are widely spaced, and are not held together at all the particles of a gas are flying around very rapidly, at about 1000 km/h Atoms and Molecules are the Particles described in the Particle Theory of Matter An atom is the smallest particle of matter Atoms cannot be divided into simpler parts by any physical or chemical method This is what John Dalton thought that an atom looked like: This is what we now think an atom looks like: A Molecule is made up of two or more atoms, combined chemically. By combining together different arrangements of the slightly more than 100 different kinds of atoms which are known to exist, every kind of substance in the entire universe can be made. (Certain special rules determine which atoms can or cannot be combined together) This is what a model of a water molecule looks like, with one oxygen atom, and two hydrogen atoms: Democritus, a philosopher in ancient Greece, over 2000 years ago, figured out that matter must be made of particles, and he called the smallest of these particles, the atom About 1800 year later, an Englishman, John Dalton, came up with more or less the same idea. He said that atoms were extremely tiny, indivisible, and indestructible, but he also said that all the atoms of a certain element had the same mass. He also said that different kinds of elements were made up of atoms of different masses. Since Dalton lived about 200 years ago, today's scientists know a lot more about atoms, and how they are made up, than he did. However, his idea of what atoms are like, makes it easy for us to see how molecules are made up. Elements Elements are substances made up of only one kind of atom. Elements, therefore, cannot be broken down into any simpler substances by physical or chemical methods. Examples of commonly-known elements are oxygen, carbon, hydrogen, iron, copper, silver, and gold. The four most important elements in the human body are carbon (C), oxygen (O), hydrogen (H), and nitrogen (N) The letters in the brackets in the line above are called symbols. These are the correct, official, abbreviations for the names of these elements. Here are some more symbols: iron is Fe, copper is Cu, silver is Ag, gold is Au, sodium is Na, and chlorine is Cl . The first letter in any element's symbol is always capitalized. The second letter (if there is one) is never capitalized. Compounds are substances made up of at least two different kinds of elements, combined chemically. Compounds cannot be broken down by physical methods, but they can be broken down by chemical methods. Commonly-known compounds are water (H20), cane sugar (C12H22O11), and table salt (NaCl) In brackets in the line above, are shown the formulas of each of those compounds. A formula is the correct, official abbreviation for the name of a compound, which also shows how the molecules of that compound are put together. You can recognize the symbols of the elements that make up those compounds. The small, subscripted number after an element's symbol in the formula tells how many atoms of that element there are, in one molecule of that compound.