Chapter 17
advertisement

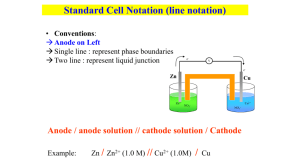
John E. McMurry • Robert C. Fay C H E M I S T R Y Chapter 17 Electrochemistry Galvanic Cells 2 CuSO4 (aq) + Zn(s) ------>ZnSO4 (aq) + Cu(s) As this spontaneous reaction proceeds, the Zn(s) dissolves, Cu precipitates. This a redox reaction: Net ionic equation: Cu2+ (aq) + Zn (s) ----> Cu (s) + Zn2+ (aq) This can be split into two half reactions: Cu2+ (aq) + 2e- Cu (s) reduction (gain of electrons) Zn (s) Zn2+ (aq) +2e- oxidation (loss of electrons Electrochemistry: The area of chemistry concerned with the interconversion of chemical and electrical energy Galvanic (Voltaic) Cell: A spontaneous chemical reaction which generates an electric current Electrolytic Cell: An electric current which drives a nonspontaneous reaction Electrochemical Cell Components Two conductors (anode and cathode) Electrolytes solution: solution that each electrode is emerse in it External circuit: provide a pathway for electron to move from one electrode to another Salt Bridge: provide neutrality Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) Galvanic Cells • Anode: • The electrode where oxidation occurs. • The electrode where electrons are produced. • Is what anions migrate toward. • Has a negative sign. Anode (-) Cathode (+) •Cathode: •The electrode where reduction occurs. •The electrode where electrons are consumed. •Is what cations migrate toward. •Has a positive sign Galvanic Cells • Salt Bridge: a U-shaped tube that contains a gel permeated with a solution of an inert electrolytes • Maintains electrical neutrality by a flow of ions • Anions flow through the salt bridge from the cathode to anode compartment • Cations migrate through salt bridge from the anode to cathode compartment Why do negative ions (anions) move toward the negative electrode (anode)? Shorthand Notation for Galvanic Cells or Voltaic Cell Salt bridge Anode half-cell Cathode half-cell Zn(s) | Zn2+(aq) || Cu2+(aq) | Cu(s) Electron flow Phase boundary Phase boundary Shorthand Notation for Galvanic Cells or Voltaic Cell How do you write the line-notation for a galvanic cell that has an aqueous or gaseous component instead of a solid metal? Cell involving gas Additional vertical line due to presence of addition phase List the gas immediately adjacent to the appropriate electrode Detailed notation includes ion concentrations and gas pressure Pt(s)|Fe2+(aq), Fe3+(aq)||Ag+(aq)|Ag(s) Example Consider the reactions below ◦ Write the two half reactions ◦ Identify the oxidation and reduction half ◦ Identify the anode and cathode ◦ Give short hand notation for a galvanic cell that employs the overall reaction Fe (s) + Sn2+ (aq) Fe2+ (aq) + Sn (s) Example Given the following shorthand notation, sketch out the galvanic cell Pb (s) | Pb2+ (aq) || Br2 (l) | Br- (aq) | Pt (s) Cell Potentials and Free-Energy Changes for Cell Reactions Electromotive Force (emf): The force or electrical potential that pushes the negatively charged electrons away from the anode ( electrode) and pulls them toward the cathode (+ electrode). It is also called the cell potential (E) or the cell voltage. The standard hydrogen electrode (S.H.E.) has been chosen to be the reference electrode. 2H+(aq, 1 M) + H2(g, 1 atm) 2e H2(g, 1 atm) 2H+(aq, 1 M) + 2e E°ox = 0 V E°red = 0 V Standard Reduction Potentials Eocell is the standard cell potential when both products and reactants are at their standard states: ◦ Solutes at 1.0 M ◦ Gases at 1.0 atm ◦ Solids and liquids in pure form ◦ Temp = 25.0oC E°cell = E°ox + E°red Standard Reduction Potentials Anode half-reaction: H2(g) Cu2+(aq) + 2e Cathode half-reaction: H2(g) + Cu2+(aq) Overall cell reaction: 2H+(aq) + 2e Cu(s) 2H+(aq) + Cu(s) E°cell = E°ox + E°red 0.34 V = 0 V + E°red A standard reduction potential can be defined: Cu2+(aq) + 2e Cu(s) E° = 0.34 V Standard Reduction Potentials Spotaniety of the reaction can be determined by the positive Eocell value The cell reaction is spontaneous when the half reaction with the more positive Eo value is cathode Note: Eocell is an intensive property; the value is independent of how much substance is used in the reaction Ag+(aq) + e- Ag(s) 2 Ag+(aq) + 2e- 2 Ag(s) Eored = 0.80 V Eored = 0.80V Standard Reduction Potentials Examples Of the two standard reduction half reactions below, write the net equation and determine which would be the anode and which would be the cathode of a galvanic cell. Calculate Eocell a. b. Cd2+(aq) + 2e- Cd(s) Eored = -0.40 V Ag+(aq) + e- Ag(s) Eored = 0.80 V Fe2+(aq) + 2e- Fe(s) Eored = -0.44 V Al3+(aq) + 3e- Al(s) Eored = -1.66 V Cell Potentials and Free-Energy Changes for Cell Reactions faraday or Faraday constant The electric charge on 1 mol of electrons and is equal to 96,500 C/mol e DG = nFE Free-energy change or DG° = nFE° Cell potential Number of moles of electrons transferred in the reaction Cell Potentials and Free-Energy Changes for Cell Reactions The standard cell potential at 25 °C is 1.10 V for the reaction: Zn(s) + Cu2+(aq) Zn2+(aq) + Cu(s) Calculate the standard free-energy change for this reaction at 25 °C. Is the reaction spontanous at this condition? Examples Calculate the cell potential at standard state (Eocell) for the following reaction. Then write the half reactions I2(s) + 2 Br-(aq) 2I-(aq) + Br2(l) ΔGo = 1.1 x 105J Standard Cell Potentials and Equilibrium constants Nernst Equation: describe the relationship between Ecell and the concentration of species involved in the cell reaction 0.0592 V E = E° log Q n At Equilibrium E = 0 E° = 0.0592 V n log K in volts, at 25 oC Standard Cell Potentials and Equilibrium Constants Example What is the value of Eo for a redox reaction involving the transfer of 2 mol electrons if its equilibrium constant is 1.8 x 10-5? Example Calculate the concentration of cadmium ion in the galvanic cell below Cd(s)|Cd2+(aq)(?M)||Ni2+(aq)(0.100M)|Ni(s) Ecell = 0.30V Electrolysis and Electrolytic Cells Anode: where oxidation takes place ◦ Anions are oxidized at this electrode ◦ labeled positive to reflect anions attraction to anode Cathode: where reduction takes places ◦ Cations are reduced at this electrode ◦ Labeled negative to reflect the cations attraction to cathode Electrolysis and Electrolytic Cells Electrolysis: The process of using an electric current to bring about chemical change. Electrolysis and Electrolytic Cells • Electrolysis: The process of using an electric current to bring about chemical change. • Process occurring in galvanic cell and electrolytic cells are the reverse of each other • In an electrolytic cell, two inert electrodes are dipped into an aqueous solution Predicting the Products of Electrolysis – The cations (+) are attracted to the cathode (-) and the anions (-) are attracted to the anode (+) Electrolysis of molten salts – used for industrial isolation of the most active elements (Na, Li, Mg, Al, …; F2, Cl2, Br2, …) – The cation is reduced at the cathode – The anion is oxidized at the anode Example: Isolation of Na and Cl2 by electrolysis of molten NaCl Na+(l) + e- → Na(l) (×2) cathode, reduction 2Cl-(l) → Cl2(g) + 2e- anode, oxidation 2Na+(l) + 2Cl-(l) → 2Na(l) + Cl2(g) net-ionic equation Electrolysis of Molten Salts Write the half-reactions for the electrolysis of the following molten compounds KCl(s) MgO(s) Electrolysis of mixed molten salts – The cation with higher Eo value (the stronger oxidizing agent) is reduced at the cathode – The anion with lower Eo value (the stronger reducing agent) is oxidized at the anode Example: Predict the products of the electrolysis of a molten mixture of NaCl and AlF3 → Possible cathode half-reactions (reduction) 1) Reduction of Na+ and 2) Reduction of Al3+ Possible anode half-reactions (oxidation) 1) Oxidation of F- and 2) Oxidation of Cl-