Chapter 3: The Molecules of Cells
advertisement

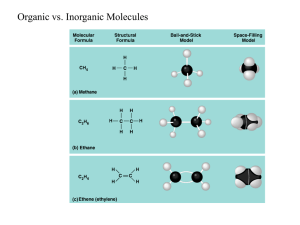
Chapter 3 The Molecules of Cells By Dr. Par Mohammadian Overview: -Carbon atom -Functional Groups -Major Biomolecules Life’s molecular diversity is based on the properties of carbon – Cell consists mostly of carbon-based molecules – Organic chemistry: Study of carbon compounds Carbon: – It has four electrons in an outer shell that holds eight – Carbon can share its electrons with other atoms to form up to four covalent bonds • The simplest organic compounds are hydrocarbons – These are organic molecules containing only carbon and hydrogen atoms – The simplest hydrocarbon is methane Functional groups help determine the properties of organic compounds • The unique properties of an organic compound depend not only on its carbon skeleton but also on the atoms attached to the skeleton – These atoms are called functional groups • Functional groups are the groups of atoms that participate in chemical reactions – Hydroxyl groups are characteristic of alcohols – The carboxyl group acts as an acid Cells make a huge number of large molecules from a small set of small molecules • Most of the large molecules in living things are macromolecules called polymers (e.g. proteins, DNA) – Polymers are long chains of smaller molecular units called monomers – A huge number of different polymers can be made from a small number of monomers • Cells link monomers to form polymers by dehydration synthesis • Polymers are broken down to monomers by the reverse process, hydrolysis BIOLOGICAL MOLECULES • There are four categories of large molecules in cells – Carbohydrates – Lipids – Proteins – Nucleic acids Carbohydrates • Carbohydrates include – Small sugar molecules in soft drinks – Long starch molecules in pasta and potatoes Monosaccharides are the simplest carbohydrates • Monosaccharides • The are simple sugars monosaccharides glucose and – Their atoms are fructose are arranged differently isomers Disaccharides • A disaccharide is a double sugar • Disaccharides are joined by the process of dehydration synthesis – It is constructed from two monosaccharides • The most common disaccharide is sucrose, common table sugar – It consists of a glucose linked to a fructose – Sucrose is extracted from sugar cane and the roots of sugar beets • Simple sugars and double sugars dissolve readily in water – They are hydrophilic Polysaccharides • Complex carbohydrates are called polysaccharides – They are long chains of sugar units – They are polymers of monosaccharides • These large molecules are polymers of hundreds or thousands of monosaccharides linked by dehydration synthesis • Starch and glycogen are polysaccharides that store sugar for later use • Cellulose is a polysaccharide in plant cell walls Lipids • Lipids are hydrophobic – They do not mix with water – Examples: fats and steroids • Fats are lipids whose main function is energy storage – They are also called triglycerides • A triglyceride molecule consists of one glycerol molecule linked to three fatty acids • The fatty acids of unsaturated fats (plant oils) contain double bonds – These prevent them from solidifying at room temperature • Saturated fats (lard) lack double bonds – They are solid at room temperature Phospholipids, waxes, and steroids are lipids with a variety of functions • Phospholipids are a major component of cell membranes • Waxes form waterproof coatings • Steroids are often hormones Steroids • Steroids are very different from fats in structure and function – The carbon skeleton is bent to form four fused rings • Cholesterol: your body produces other steroids PROTEINS Proteins are essential to the structures and activities of life • Proteins are involved in – – – – – cellular structure movement defense transport communication • Mammalian hair is composed of structural proteins • Enzymes regulate chemical reactions The Monomers: Amino Acids • Each amino acid consists of – A central carbon atom bonded to four covalent partners – an amino group – a carboxyl group – A side group that is variable among all 20 • All proteins are constructed from a common set of 20 kinds of amino acids Proteins as Polymers • Cells link amino acids together by dehydration synthesis – The resulting bond between them is called a peptide bond • Your body has tens of thousands of different kinds of protein – The arrangement of amino acids makes each one different Overview: A protein’s specific shape determines its function • A protein, such as lysozyme, consists of polypeptide chains folded into a unique shape – The shape determines the protein’s function – A protein loses its specific function when its polypeptides unravel A protein’s primary structure is its amino acid sequence Secondary structure is polypeptide coiling or folding produced by hydrogen bonding Tertiary structure is the overall shape of a polypeptide Quaternary structure is the relationship among multiple polypeptides of a protein What Determines Protein Structure? • A protein’s shape is sensitive to the surrounding environment – Unfavorable temperature and pH changes can cause a protein to unravel and lose its shape – This is called denaturation NUCLEIC ACIDS Nucleic acids are information-rich polymers of nucleotides • Nucleic acids such as deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) serve as the blueprints for proteins • They ultimately control the life of a cell • Nucleic acids are polymers of nucleotides – Each nucleotide is composed of a sugar, phosphate, and nitrogenous base (RNA has A, C, G and instead of T, it has uracil (U). • Each DNA nucleotide has one of the following bases – – – – Adenine (A) Guanine (G) Thymine (T) Cytosine (C)