Quantum Numbers Worksheet I
advertisement

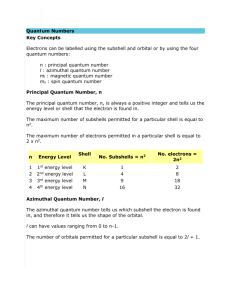
Worksheet on Quantum Numbers in Schrodinger’s Quantum Mechanical Model of Atoms 1. List all possible sets of quantum numbers for n =3 2. List all possible atomic orbitals with n = 4 3. What are the possible values of (l) if: a.) n=2 b.) n=4 4. What letters correspond with the values of (l) in #3? 5. How do you designate the orbitals when: a.) n=2, l=0 b.) n=3, l=2 c.) n=3, l=1 6. How many orbitals are there if: a.) l=2 b.) l=3 7. What letters correspond with the subshells in #6? 8. What is the maximum number of electrons found in the: a.) 1st principal energy level b.) 2nd principal energy level c.) 3rd principal energy level d.)4th principal energy level 9. How many electrons are there in: a.) a (s) subshell b.) a (p) subshell c.) a (d) subshell d.) a (f) subshell 10. How many electrons are there in: a.) a (p) orbital b.) a (f) orbital 11. How many orbitals are there in: a.) a (s) subshell b.) a (p) subshell c.) a (d) subshell d.) a (f) subshell 12. For the following pairs of orbitals, indicate which is higher in energy: a.) 3p or 4p b.) 4s or 4d c.) 2s or 3d d.) 5s or 4f 13. What type of orbital (s, p, d, or f) is designated by: a.) n=4, l=1, ml=0 b.) n=3, l=2, ml=0 c.) n=2, l=0, ml=0 14. Indicate which of the following sets of quantum numbers could NOT occur and explain why: a.) 1,1,0,+1/2 b.) 2,1,0,+1/2 c.) 2,0,1,-1/2 d.) 2,1,0,0 e.)3,2,0,-1/2