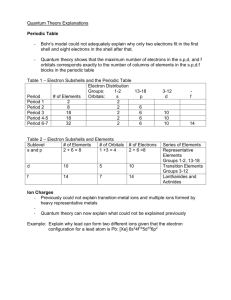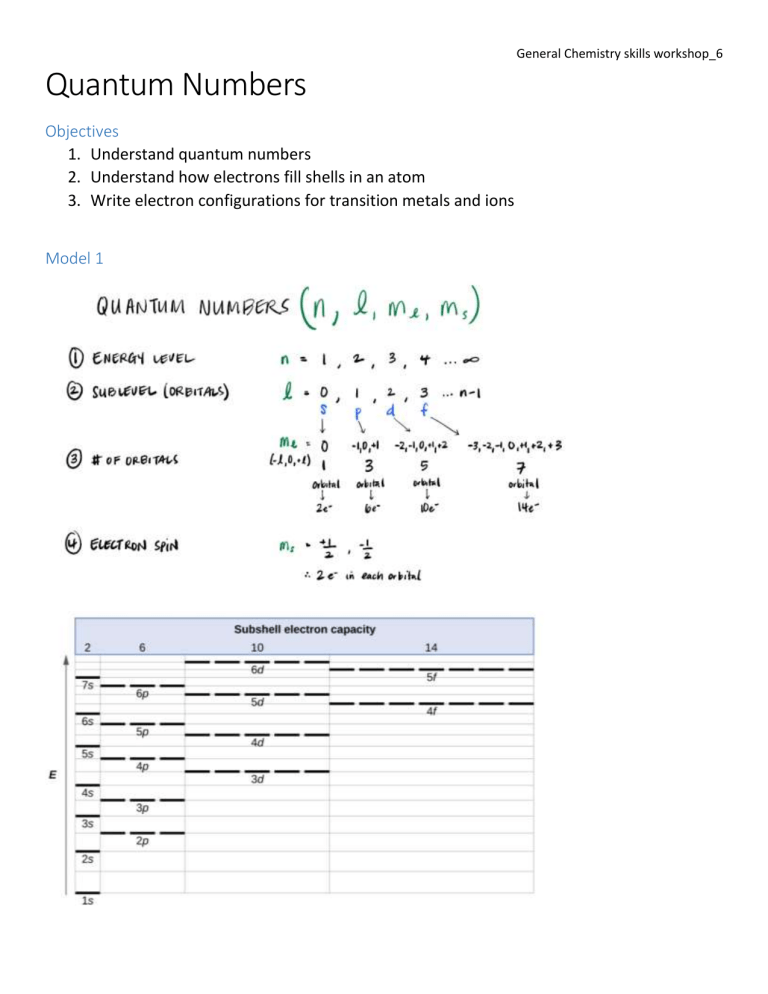
General Chemistry skills workshop_6 Quantum Numbers Objectives 1. Understand quantum numbers 2. Understand how electrons fill shells in an atom 3. Write electron configurations for transition metals and ions Model 1 General Chemistry skills workshop_6 Exploring the Model 1. What type of electron orbital (i.e., s, p, d, or f) is designated by: a. n = 2, l = 1 b. n = 4, l = 2 c. n = 6, l = 0 2. What is the maximum number of electrons the above orbitals can hold? a. n = 2, l = 1 b. n = 4, l = 2 c. n = 6, l = 0 General Chemistry skills workshop_6 Model 2 Exploring the model Each shell in the model has one or more subshells. These subshells are of 4 sizes: s, p, d and f. An s subshell then will hold a maximum of two electrons according to our model. 3. How many electrons will a p, d and f subshell hold? 4. The second figure shows a relationship between the periodic table and electron configurations. Briefly explain how we can use the periodic table to determine an atom’s electron configuration. General Chemistry skills workshop_6 Extending the model Experiment tell us, that electrons will fill the 1s subshell first. They will then fill the 2s and 2p subshell. Next, they will fill the 3s subshell, followed by the 3p. However, before going into the large five orbital 3d subshell, electrons will first fill the 4s subshell. After filling the 4s subshell, they will then proceed to fill the 3d subshell. The 4p subshell is filled next. The electrons prefer to fill the small 5s subshell before filling the larger 4d subshell. As we fill higher subshells, it takes more energy to fill them. The 1s subshell is the lowest energy! The order of filling is called the electron configuration. For example, you would write the electron configuration for Cu: 1s22s22p63s23p64s23d9 or abbreviated with the prior noble gas [Ar]4s23d9 5. What is the electron configuration of Ni? 6. What is the electron configuration of Fe? General Chemistry skills workshop_6 7. Looking at the electron configuration for Fe (from question 4) and given that the electron configuration or Fe3+ is 1s22s22p63s23p63d5 (or abbreviated [Ar] 3d5), what is unusual about this configuration (think d versus s)? 8. How many electrons in Zn have ml=0 as one of their quantum numbers? 9. How many electrons in Ni can have l=2 as one of their quantum numbers?