BIOL30001-2015-05
advertisement

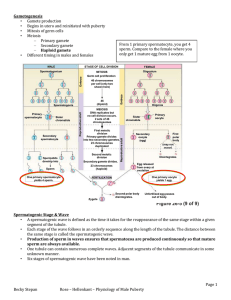
During the service, the pastor asked if anyone in the congregation would like to express thanks for prayers which had been answered. A lady stood up and came forward. She said, "I have a reason to thank the Lord. Two months ago, my husband, Jim, had a terrible bicycle wreck and his scrotum was completely crushed. The pain was excruciating and the doctors didn't know if they could help him." You could hear an audible gasp from the men in the congregation as they imagined the pain that poor Jim experienced. She continued, "Jim was unable to hold me or the children and every move caused him terrible pain. We prayed as the doctors performed a delicate operation. They were able to piece together the crushed remnants of Jim's scrotum and wrap wire around it to hold it in place." Again, the men in the Congregation squirmed uncomfortably as they imagined the horrible surgery performed on Jim. She continued, "Now, Jim is out of the hospital and the doctor's say, with time, his scrotum should recover completely." All the men sighed with relief. The pastor rose and tentatively asked if any one else had anything to say. A man rose and walked slowly to the podium. He said, "Hi, I'm Jim and I would like to tell my wife, the word is 'sternum.' " The real story of the snake in the Garden of Eden … Dictionary definitions directile disfunction: the inability of males, when “geographically embarrassed” (lost), to stop and ask for directions. BIOL30001 Reproductive Physiology Spermatogenesis and testicular endocrinology Geoff Shaw Reading: EssRepro7 Chapters 1, 6, 7 Sharpe (1994) Regulation of spermatogenesis. In Knobill & Neill. The Physiology of Reproduction vol 2 Setchell B (1982) Spermatogenesis and spermatozoa. In Reproduction in Mammals V1, Eds Austin CR & Short RV Knobill & Neill. (eds) (1994) The Physiology of Reproduction vol 2, chaps 20-22 Endotext.com and most good endocrinology or embryology books In summary testis (sperm production) epididymis (sperm transport, maturation and ejaculation) efferent ducts (transport of sperm) vas deferens bladder Cowper’s gland (secretes a cleansing/ lubricating solution) (sperm transport and ejaculation) seminal vesicles (supply the seminal fluid) urethra prostate gland (passage for semen and urine) (secretes an alkaline neutralising solution) penis (for copulation) Sperm morphology fish amphibian birds mammal Sperm stained with fluorescent dyes that highlight head, midpiece and tail structure of a sperm Please refer to the corresponding figure in your textbook J&E fig 4.6 Johnson & Everitt 2000 fig 4.6 (see 6.7) sperm midpiece and tail Johnson & Everitt 2000 fig 4.6 entiation cytodiffer- meiosis mitosis spermatogenesis sperm development (human) residual body DeKretser & Kerr (1994) Fig 61 LUMEN spermatid secondary spermatocyte primary spermatocyte Sertoli cells peritubular myoid cells dark A spermatogonium pale A type B spermatogonium spermatogonia blood-testis barrier spermatogenic epith cycle different tubule sections have diferent appearance – a different cohort of spermatogenic stages- due to spermatogenic wave RAT see Johnson & Everitt Figs 4.8 and 4.9 Spermatogenic cycle species time for completion duration of cycle of the spermatogenesis (days) seminif. epithel. (days) man 64 16….. bull 54 13.5.. ram 49 12.25 boar 35 8.5 rat 48 12…. The duration of the spermatogenic cycle is constant and characteristic for each species J&E table 4.1 spermatogenic wave on rat seminiferous tubule As you go along the seminiferous tubule you find the sequence of spermatogenic stages in human tubule you get patches of spermatogenic initiation see Johnson & Everitt 2000 fig 4.10 (6.11) hypophysectomy stops steroidogenesis and spermatogenesis testis of normal rat testis of hypophysectomised rat FSH and Sertoli cell • FSH receptor in basal cell membrane • acts via cAMP and Ca++ • acts with T to support spermatogenesis • stimulates – – – – – – – – FSH mRNA and protein synthesis glucose transport lactate production inhibin ABP transferrin aromatase mitosis (in immature S.C.) Blood testicular steroidogenesis Sertoli cell acetate cholesterol testosterone P450scc pregnenolone DHEA androstenediol 3-HSD, 4-5-isomerase progesterone androstenedione testosterone Leydig cell Blood HO 3-HSD O ABP 5-dihydro- ABPT testosterone oestradiol androstenedione oestrone Tubule Lumen LH and control of testicular steroidogenesis LDL-cholesterol Blood Sertoli cell LH acetate LDL-Tr + cholesterol cholesterol stores LHR cAMP + testosterone ABP ABPT P450scc 5-dihydrotestosterone pregnenolone testosterone Leydig cell Blood Tubule Lumen hypothalamo-pituitary-gonadal axis GnRH GnRH - inhibin testosterone LH FSH • inhibin from Sertoli cells specifically inhibits FSH secretion by pituitary gonadotropes LH FSH • testosterone injection suppresses LH secretion • pulse frequency • pulse amplitude • in rodents DHT has same effect as T LH is essential for Leydig cell steroidogenesis hypophysectomy GnRH GnRH GnRH testosterone LH Anti-GnRH antiserum FSH testosterone LH Anti-LH antiserum testosterone FSH reversed by exogenous LH or hCG • LH receptors present on Leydig cells • LH acts primarily via cAMP • raising cAMP increases LC steroidogenesis LH FSH Control of Sertoli cell function hypophysectomy GnRH testosterone immunization against GnRH and pure FSH GnRH LH FSH testosterone hypophysectomy and high dose testosterone GnRH LH testosterone LH FSH FSH spermatogenesis halted accessory sex glands regress partial spermatogenesis resumed (block at A3-A4) spermatogenesis resumed at reduced level ASG regressed ASG re-develop Summary • Spermatogenesis – control by Sertoli cells – Spermatogonia mitotic + meiotic differentiation • • • • Spermatogonia Spermatocytes Spermatids Sperm – Spermatogenic cycle – Spermatogenic wave • Hormone production – – – – androgen – Leydig cells Testosterone Sertoli cells E2, DHT inhibin – Sertoli cells feedback control via hypothalamo-pituitary axis (HPA) Other control of testis? • secretagogues in circulation or made in testis (eg CRF, opiates, catecholamines etc) can alter Leydig cell activity independently of gonadotrophins • splanchnic nerves regulates LH receptors and testicular blood flow • injection of IL1, CRH or -adrenergic agonists reduces testicular response to exogenous hCG see Campos et al (1993) Neuroendocrinology 57:189 Dufau ML (1988) Annu Rev Physiol 50:483 role of oestrogen in spermatogenesis ER-knockout disrupts spermatogenesis and causes infertility in males probably by disrupting fluid resorption in efferent ducts from: Eddy et al (1996) Endocrinology 137:4796-4805 neuroendocrine control of the testis GnRH GnRH testosterone inhibin testosterone inhibin LH FSH castrate – about 10ng/ml, pulses every hour to 14 ng/ml intact about 1 ng/mlpulses every 2 h to 4 ng/ml LH FSH HPG axis in ram castrated rams (wethers) + T, DHT, E2 or control GnRH T,DHT,E2 or oil LH FSH GnRH infusion T,DHT,E2 or oil all treatments: LH, FSH pulse frequency hypothalamo-pituitary (HPD) disconnected wethers with GnRH pulses infused every 2 h LH FSH all treatments: no effect on LH or FSH wethers - portal blood samples for GnRH ± testosterone GnRH T or oil LH FSH effect of T: GnRH conc., GnRH pulse frequency GnRH pulse amplitude based on Tilbrook AJ, et al. (1991) Endocrinology 129:3080-92 direct neural control of testicular testosterone production? • injecting transganglionic retrograde nerve tracer into testis marks spinal cord, brain stem and hypothalamus SPINAL CORD TRANSSECTION CONTROL hCG hCG/icv CRF hCG/icv CRF testosterone testosterone hCG vehicle 0 60 time (mins) vehicle 0 60 time (mins) • effect not due to changes in endogenous LH secretion or testicular blood flow Lee, Miselis & Rivier (2002) Endocrinology 143: 4447-54 Selvage et al. (2004) Endocrinology 145: 1750-9 round spermatids to elongated spermatids spermatocytes to round spermatids spermatogonia to spermatocytes rats intact or hypophysectomised and given control implant or testosterone implant 3cm or 10 cm long for 13 weeks intact hypophysectomised from Sun et al (1990) Endocrinology 127:1215