3) What is half-life?
advertisement
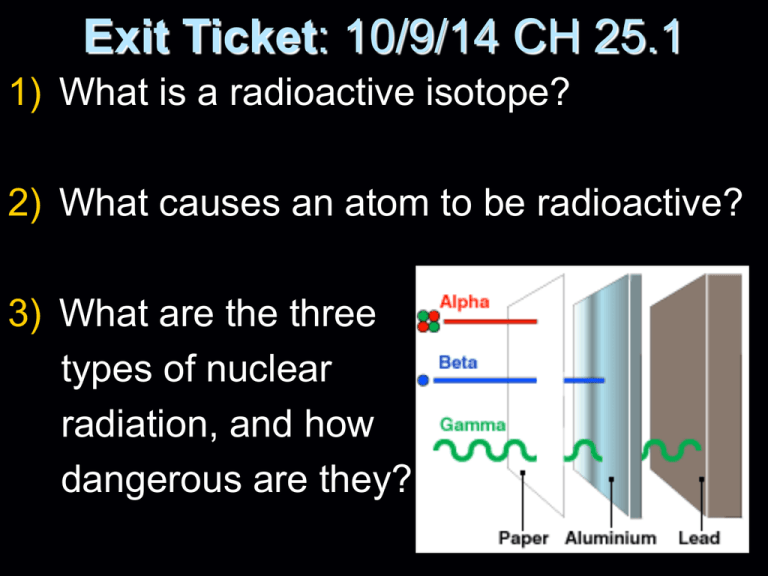
Exit Ticket: 10/9/14 CH 25.1 1) What is a radioactive isotope? 2) What causes an atom to be radioactive? 3) What are the three types of nuclear radiation, and how dangerous are they? Entrance Ticket: 10/10/14 NUCLEAR CHEMISTRY CH 25.1 1) Explain what an alpha particle is. 2) If a radioactive atom (radioisotope) emits an alpha particle, what would the nucleus be losing? 3) What is a beta particle? How is a beta particle emitted from the nucleus? Answers 1) Explain what an alpha particle is: particle made up of 2 protons & 2 neutrons (Helium nucleus) 2) If a radioactive atom (radioisotope) emits an alpha particle, what would the nucleus be losing: 2 protons & 2 neutrons 3) What is a beta particle? How is a beta particle emitted from the nucleus: high speed electron emitted from the nucleus when a neutron turns into a proton. Entrance Ticket: 10/11/14 1) Write out the nuclear reaction of the alpha decay of Radon 226. 2) What radioisotope alpha decays into Lead 209? 3) Write out the beta decay of Carbon 14. Answers to 25.1 worksheet 1.) radioactive 2.) radioisotopes 3.) nuclei 4.) stable 5.) energy 6.) beta 7.) Alpha 8.) helium 9.) electrons 10.) metal foil 11.) Gamma 12.) mass 13.) Lead/concrete 14.) Lead/concrete 15.) stop 16.) ST 17.) NT 18.) AT 19.) NT 20.) AT 21.) b 22.) a 23.) c 24.) e 25.) d Entrance Ticket 4/22/15 Nuclear Chem. Review and ½ life: 1) What is a radioactive isotope? (p. 799) 2) What causes an atom to be radioactive? 3) What is half-life? (pp. 804-806) Entrance Ticket 4/22/15 1) What is a radioactive isotope? An element that has an unstable nucleus 2) What causes an atom to be radioactive? The nucleus is too big or the ratio of protons to neutrons is unfavorable. 3) What is half-life? The amount of time it takes half of a radioactive sample to decay into products practice 1) Manganese-56 is a beta particle emitter with a half-life of 2.6 hours. a) Write out this nuclear reaction. b) Calculate how much Manganese56 you would have remaining after 10.4 hours if you had 30 grams to start with. 2) Thorium-234 has a half-life of 24.1 days. Will all of the thorium undergo radioactive decay in 48.2 days? Explain. Please get out your half-life lab (p.40) and tear out a piece of graph paper Half Life Lab -Half life is the amount of time it takes for half of a radioactive sample to decay. We will be using pennies to represent a sample of a radioactive isotope. Each time you dump the pennies, that represents a half life. Entrance Ticket 4/23/15 1) Please write out the nuclear reaction for the alpha decay of Thorium-230. 2) A radioisotope has a half-life of 20 seconds. How much of a 100 gram sample remains after 1 minute? Half Life Review 1) If a radioisotope has a half life of 3.5 hours, how many half lives has it gone through in 10.5 hours 2) Using #1, how much of an 18 gram sample would remain radioactive after 7 hours? 3) Using #2, how much time has passed if 1.125 grams of the original sample remains? Half Life Review Answers 1) The radioactive sample would go through 3 half lives. 2) After 7 hours, 4.5 grams would still be radioactive. 3) 14 hours has past if 1.125 grams are still radioactive. Review 4/27/15 1) Please write out the nuclear reaction of the alpha decay of Bismuth-214. 2) If Copper-66 becomes Zinc-66 after it has gone through radioactive decay, what type of decay did it emit? Half Life Review 1) If a radioisotope has a half life of 1.5 hours, how many half lives has it gone through in 4.5 hours? 2) How much of an 20 gram sample would remain radioactive after decaying for 4.5 hours if its half life is 1.5 hours? 3) Using #2, how much time has passed if 1.25 grams of the original sample remains? Half Life Review 1) 3 half lives 2) 2.5 grams 3) 6 hours Nuclear Fission and Fusion Describe the process of nuclear fission and nuclear fusion. How does the energy released compare to a chemical reaction? Which nuclear reaction releases more energy? Reading….A Murder Mystery 1) Do you think Alexander Litvinenko was murdered? Why? 2) What killed Alexander Litvinenko? 3) Who is responsible for his death? 4) What is the motive? Nuclear Chemistry wrap up (on e.t.) Please write a 6-8 sentence paragraph about nuclear chemistry. Use the knowledge that you now have about radioisotopes, radiation, half-life, fission and fusion. You have the freedom to focus on whatever you like, as long as it is about concepts we have learned in nuclear chem. Make sure to have a topic sentence, concrete details and a concluding sentence. Acid Base Titration Lab Reaction #1: HCl(aq) + NaOH(aq) NaCl (aq) + H2O(l) Reaction #2: H2SO4 (aq) + NaOH(aq) Na2SO4(aq)+ H2O(l) Titration Lab Reaction #2: H2SO4 (aq) + NaOH(aq) Na2SO4(aq)+ H2O(l)






