9 - Naming Chemical Compounds
advertisement

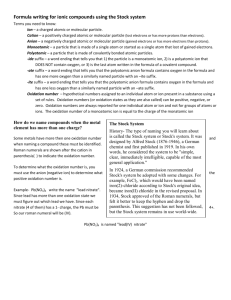
Writing Formulas for Simple Binary Compunds If the compound’s name ends in “ide” chances are it’s a binary compound. (There are a few exceptions which will be covered later). Find the 1st element on the periodic table and determine its oxidation number. If there is more than 1 oxidation state then go on to the section entitled Writing formulas of Complex Binary Compounds. Oxidation numbers can be determined from position in the periodic table below. 1 2 3 2 2 3 4 4 5 6 -4 -3 -2 3 5 7 -1 Writing Formulas for Simple Binary Compunds Find the oxidation state of the 2nd element remembering the first element is always positive and the second is always negative. Once both oxidation numbers are determined the goal is to combine the positives and the negatives in ratios where the total charge adds up to zero. Example 1 - magnesium nitride. 2+ Mg Since 3 particles have a charge 3of 6+ and 2 N particles have a charge of 6- the total charge adds up to zero. The correct formula must be: Mg2+ N3- Mg2+ N3- Mg2+ 2 N 3- particles 3 Mg 2+ particles Mg3N2 Writing Formulas for Simple Binary Compunds Example 2 - sodium phosphide. Sodium is in group 1 so its oxidation number is 1+. Phosphide is short for phosphorus. Its oxidation number must be negative since it’s the 2nd name so phosphide has a charge of 3-. If the total charge is going to add Na1+ P3up to zero 3 Na1+ and 1 P3- must be used. Na1+ Na1+ The final answer is: Na3P Writing Formulas for Complex Binary Compounds - “ous,ic” If the 1st name ends in “ous” or “ic” find the element on the periodic table. Latin names are sometimes used. Below is a list of the latin names most frequently used: ferrous, 2+ ferric - 3+ cuprous, 1+ cupric - 2+ stannous, 2+ stannic - 4+ plumbous, 2+plumbic - 4+ iron coppertin lead - Fe 29Cu 50Sn 82Pb 26 Generally “ous” is used for the lowest positive oxidation state and “ic” is the next highest. There are exceptions to this rule. Example - Stannous chloride Cl1- Sn2+ Cl1- SnCl2 Example - plumbic sulfide 4+ Pb 2S - 2S - PbS2 Writing Formulas for Complex Binary Compounds Roman Numeral System - IUPAC When names for compounds have Roman Numerals present, this number represents the charge of the positive ion. This charge can be used to determine the number of negative particles needed to create a combination of particles with an overall charge of zero. Example sulfur(VI) oxide S6+ O2- O2- O2- SO3 The Roman Numeral VI stands for 6. (V is 5 and I after it means add 1 to 5. The Roman Numeral for 4 is IV) If sulfur particles have a charge of 6+, 3 oxygen particles, each with a charge of 2- are needed to create a collection of particles where the total charge adds up to zero. Writing Formulas for Complex Binary Compounds - Prefix System Prefix monoditritetrapentahexaheptaocta- No. of Atoms When a compound ending in “ide” also contains prefixes 1 like mono, di, tri, etc. the 2 formula can be written using 3 these prefixes instead of 4 using charges. 5 Example - dicarbon tetrahydride 6 2 carbons 4 hydrogens 7 8 C2H4 Writing Formulas of Oxyacids The “ic” acids O3 nitric acid H2 C O3 carbonic acid H 1 Cl O3 chloric acid H2 S O4 sulfuric acid H3 P O4 phosphoric acid H1 N Writing Formulas of Oxyacids - “ous” and “ic” Per_____ic _____ ic ______ ous HNO4 HNO3 HNO2 H2CO4 H2CO3 H2CO2 H2CO HClO4 HClO3 HClO2 HClO H2SO5 H2SO4 H2SO3 H2SO2 H3PO5 H3PO4 H3PO3 H3PO2 add 1 O remove 1 O, hypo ____ ous HNO remove 2 O’s Family Substitutions Since elements in the same family have the same number of valence shell electrons they can sometimes be freely substituted for one another. Some examples of family substitutions are: F P S Cl As Se Br Te I Po phosphate arsenate sulfite selenite PO43AsO43SO32SeO32- hypochlorite hypofluorite hypobromite ClO1FO1BrO1- perchoric acid HClO4 HBrO4 perbromic acid Deriving Polyanions From the Oxyacids Each of the oxyacids below is made up of positive H ions and negative polyanions HNO3 H1+ NO31- nitrate H2CO3 H1+ CO32- H1+ carbonate HClO3 H1+ ClO 13 chlorate H2SO4 H1+ SO42- H1+ sulfate H3PO4 H1+ PO43- H1+ H1+ phosphate Deriving “ites” from “ates” per_____ate _____ ates ______ ites hypo ____ite NO41- NO31- NO21- NO1- CO42- CO32- CO22- CO2- ClO41- ClO31- ClO21- ClO1- SO52- SO42- SO32- SO22- PO53- PO43- PO33- PO23- add 1 O remove 1 O, remove 2 O’s Writing Formulas - “ates” & “ites” Example 1 - aluminum carbonite Determine the charges of each particle Al3+ CO22Al3+ CO22- CO22- The answer is Al2(CO 6- 2)3 6+ zero charge Compile groups of particles where the sum of positive charges equals the sum of negative particles. This way the total charge is zero. 2 Al 3+ equals 6+ and 3 CO2 2- equals 6-. Naming “ates” & “ites” Example 1 - Fe(NO3)2 Since the positive ion has more than one oxidation state, its specific oxidation state must be determined. This is done by figuring out the charge on the negative particle. Since the total negative charge is 2- the positive charge on the single particle of Fe must be 2+. Remember oxyacid is HNO3 2+ Fe Using the “ous” “ic” method, the name is ferrous. Using the IUPAC method the name is iron(II). NO31- is called nitrate. NO31- NO31- 2+ 2- zero charge The answer is ferrous nitrate or iron(II) nitrate Polyatomic Anions Containing H H1+ ions can be added to any of the “ates”or “ites” with a 2- or 3- charges, reducing the overall charge of the newly formed polyatomic negative ion by one. This is shown below: 12H1+ CO2 HCO2 hydrogen carbonite carbonite or bicarbonite 1+ If 2 H ions are added to any of the “ates”or “ites” with a 3- charge the resulting particle is named: H1+ PO43- H1+ phosphate 1- H2PO4 dihydrogen phosphate Polyatomic Anions Containing H Write the formula for manganese (III) dihydrogen phosphite All formulae in this course consists of a positive particle (cation) and a negative particle (anion). The positive particle is Mn3+. This is determined from the Roman Numeral in the name. 3- 1- H PO 1- H PO 1Mn3+ H PO PO 2 3 3 2 3 2 3 The negative particle is a dihydrogen phosphite. Phosphite i After adding 2 hydrogens to the phosphite the charge decreases to 1- and the anion becomes: To balance charges 3 of these dihydrogen phosphites are needed. The resulting answer is: Mn(H2PO3)3 Water is Special Polyatomic Ions 121+ 1+ OH H O H In some instances one of these H1+ particles is hydroxide lost. The anion OH1- is formed. This particle is called hydroxide. Vinegar is a 5% solution of acetic acid. Its stucture is shown below. Sometimes it loses a H1+ particle to water forming: H H C H H C O O H H C2H3O21- HC2H3O2 C H C O O acetate Special Polyatomic Ions Sulfur is in group VIB and sulfate has the formula SO42-. Chromium is in group VIA and chromate has the formula CrO42chromate O O Cr O O If two of these chromate particles are combined one of the oxygen atoms is lost forming: O O O O dichromate Cr 2O O Cr O CrO OCr Cr OO O 2 7 O O O O Special Polyatomic Ions Cyanide salts used in execution chambers in the U.S. are combined with sulfuric acid producing poisonous hydrogen cyanide gas. The anion cyanide has the formula: CN1cyanide cyanate salts contain the anion CNO1cyanate The prefix “thio” is used whenever an O particle is replaced by a sulfur particle since both O and S are in the same family. The anion thiocyanate has the formula: CNO S 1thiocyanate Thiosulfate has the formula: SO42- S2O32- Special Polyatomic Ions Manganese is in group VIIA and chlorine is in group VIIB. This allows some creative substitution. Chlorate has the formula ClO31- perchlorate chlorate is 1- permanganate ClO4 perchlorate is MnO41permanganate A poisonous substance found in the leaves of rhubarb, potatoes, tomatoes and countless other plants is called oxalic acid. Its formula is H2C2O4 If this acid loses two H1+ particles it creates the anion oxalate. C2O42oxalate Special Polyatomic Ions The poisonous gas, nitrogen trihydride, NH3, has the common name ammonia. ammonia When ammonia molecules dissolve in water and collide with water molecules they sometimes form Notice the water molecule left a H1+ particle behind forming N H H H H O H O HH O H ammonium - NH N H H H 4 1+ hydroxide OH1- cyanide CN1- oxalate C2O42- acetate C2H3O21- cyanate CNO1- oxalate C2O42- chromate CrO41- thiosulfate S2O32- ammonium NH41+ dichromate Cr2O72- permanganate MnO41- Naming Chemical Compounds Yes Are there more than 2 elements? It has polyatomic ions. Does the negative ion come from an oxyacid? Yes No Use the HO, HO table to determine its name. It must be a special polyatomic ion. No It is a binary compound so the name must end in ide. Does the 1st element have more than 1 oxidation state? Yes No Determine the oxidation state of the 1st element and use it to name the compound. Name it. Writing Formulas for Chemical Compounds No Does the name end in “ide”? It’s made up of polyatomic ions. Does the negative ion come from an oxyacid? Yes Use the HO, HO table to determine its formula. No It must be a special polyatomic ion. Yes It is probably a binary compound. Does the 1st element have more than 1 oxidation state? Yes No Determine the oxidation state of the Determine 1st element and use it the formula. to determine the formula. Binary Acids Hydrochloric acid HCl Hydrobromic acid HBr Hydroiodic acid HI Hydrofluoric acid HF Hydrosulfuric acid H2S Hydroselenic acid H2Se