1 mole O
advertisement

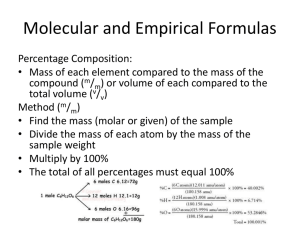
% Composition or %Mass Percent Composition: the mass of each element, in percent, that makes up a compound % composition = part/whole x 100 % Composition or % Mass % Composition: part/whole x 100 % mass = g desired element X 100 total g of compound Make sure the % add up to 100 % Don’t confuse % Composition with Molar Mass % mass = g desired element X 100 = % total g of compound Molar Mass = atomic mass from periodic table = g/mol Determine the percentage composition of the following compound HNO3 Determine the percentage composition of the following compound HNO3 has 1 mol H = 1 mol N = 3 mol O = Determine the percentage composition of the following compound HNO3 has 1 mol H = 1.01 g 1 mol N = 14.01 g 3 mol O = 48.00 g 63.02 g HNO3 Next we take each individual element and divide by the mass of compound Part/whole x 100 = % composition Determine the percentage composition of the following compound HNO3 has 1 mol H = 1.01 g 1 mol N = 14.01 g 3 mol O = 48.00 g 63.02 g HNO3 Next we take each individual element and divide by the mass of compound Part/whole x 100 = % composition 1.01 g H x 100 = 1.60% 63.02 g HNO3 Determine the percentage composition of the following compound HNO3 has 1 mol H = 1.01 g 1 mol N = 14.01 g 3 mol O = 48.00 g 63.02 g HNO3 Part/whole x 100 = % composition 1.01 g H x 100 = 1.60% 63.02 g HNO3 14.01 g N x 100 = 22.23% 63.02 g HNO3 Determine the percentage composition of the following compound HNO3 has 1 mol H = 1.01 g 1 mol N = 14.01 g 3 mol O = 48.00 g 63.02 g HNO3 Part/whole x 100 = % composition 1.01 g H x 100 = 1.60% 63.02 g HNO3 14.01 g N x 100 = 22.23% 63.02 g HNO3 48.00 g O x 100 = 76.17% 63.02 g HNO3 % Composition or % Mass % mass = g desired element X 100 total g of compound Make sure the % add up to 100 % If 8.20 g Mg combines with 5.40g O, what is the % of each compound? %mass Mg= % Composition or % Mass % mass = g desired element X 100 total g of compound Make sure the % add up to 100. If 8.20 g Mg combines with 5.40g O, what is the % of each compound? %mass Mg= 8.2 g Mg = 8.2 g Mg x 100=60.3% 8.2 + 5.4 13.6 g MgO % Composition % mass = g desired element X 100 total g of compound Make sure the % add up to 100. If 8.20 g Mg combines with 5.40g O, what is the % of each compound? %mass Mg= 8.2 g Mg = 8.2 g Mg x 100=60.3% 8.2 + 5.4 13.6 g %mass O = 5.4 g O = 5.4 g O x 100 = 39.7% 8.2 + 5.4 13.6 g % Composition % mass = g desired element X 100 total g of compound Make sure the % add up to 100. If 8.20 g Mg combines with 5.40g O, what is the % of each compound? %mass Mg= 8.2 g Mg = 8.2 g Mg x 100=60.3% 8.2 + 5.4 13.6 g %mass O = 5.4 g O = 5.4 g O x 100 = 39.7% 8.2 + 5.4 13.6 g 100 - 60.3= 39.7 % Composition % Mass % mass = g desired element X 100 total g of compound Calculate the % composition of C3H8 % Composition % Mass % mass = g desired element X 100 total g of compound Calculate the % composition of C3H8 C = 3(12.01) = 36.03 g C % Composition % mass = g desired element X 100 total g of compound Calculate the % composition of C3H8 C = 3(12.01) = 36.03 g C H = 8(1.01) = 8.08 g H % Composition % mass = g desired element X 100 total g of compound Calculate the % composition of C3H8 C = 3(12.01) = 36.03 g C H = 8(1.01) = 8.08 g H % mass C = 36.03 g C = 36.03 g C x 100 = 81.7% 36.03 +8.08 44.11 g % Composition % mass = g desired element X 100 total g of compound Calculate the % composition of C3H8 C = 3(12.01) = 36.03 g C H = 8(1.01) = 8.08 g H % mass C = 36.03 g C = 36.03 g C x 100 = 81.7% 36.03 +8.08 44.11 g % mass H = 8.08 g C = 8.08 g C x 100 = 18.3% 36.03 +8.08 44.11 g % Composition % mass = g desired element X 100 total g of compound Calculate the % composition of C3H8 C = 3(12.01) = 36.03 g C H = 8(1.01) = 8.08 g H % mass C = 36.03 g C = 36.03 g C x 100 = 81.7% 36.03 +8.08 44.11 g % mass H = 8.08 g C = 8.08 g C x 100 = 18.3% 36.03 +8.08 44.11 g total 100% Calculate mass C in 82.0g C3H8 C = 3(12.01) = 36.01 g C H = 8(1.01) = 8.08 g H ??????? Calculate mass C in 82.0g C3H8 C = 3(12.01) = 36.03 g C H = 8(1.01) = 8.08 g H total = 44.11 g C3H8 36.03 g x 100 = 81.7% C 44.11 g Calculate mass C in 82.0g C3H8 C = 3(12.01) = 36.03 g C H = 8(1.01) = 8.08 g H 36.03 g x 100 = 81.7% C 44.11 g 82.0 g C3H8 x 81.7% = 66.994 = 67.0 g C Find the percent composition of a compound that contains 20.4 g Nitrogen and 27.3 g Oxygen in a 47.7 g sample Find the percent composition of a compound that contains 20.4 g Nitrogen and 27.3 g Oxygen in a 47.7 g sample 20.4 g N x 47.7 g Sample 100 = Find the percent composition of a compound that contains 20.4 g Nitrogen and 27.3 g Oxygen in a 47.7 g sample 20.4 g N x 47.7 g Sample 100 = 42.8% Nitrogen Find the percent composition of a compound that contains 20.4 g Nitrogen and 27.3 g Oxygen in a 47.7 g sample 27.3 g O x 47.7 g Sample 100 = 57.2 % Oxygen Find the percent composition of a compound that contains 20.4 g Nitrogen and 27.3 g Oxygen in a 47.7 g sample 20.4 g N x 47.7 g Sample 100 = 42.8% Nitrogen 27.3 g O x 47.7 g Sample 100 = 57.2 % Oxygen 100% Find the percent composition of MgNO3 Mg = N= 3O= Find the percent composition of MgNO3 Mg = 24.31 g N = 14.01 g 3 O = 3 x 16.00 = 48.00 g O Find the percent composition of MgNO3 Mg = 24.31 g N= 14.01 g 3 O = 3 x 16.00 = 48.00 g O 86.32 g MgNO3 Find the percent composition of MgNO3 Mg = 24.31 g + N= 14.01 g + 3 O = 3 x 16.00 = 48.00 g O 86.32 g MgNO3 Find the percent composition of MgNO3 Mg = 24.31 g Mg x 100= 28.16% 86.32 g MgNO3 N= 14.01 g N x 100= 16.23% 86.32 g MgNO3 O= 48.00 g O x 100= 55.61% 86.32 g MgNO3 Find the percent composition of MgNO3 Mg = 24.31 g Mg x 100= 28.16% 86.32 g MgNO3 N= 14.01 g N x 100= 16.23% 86.32 g MgNO3 O= 48.00 g O x 100= 55.61% 86.32 g MgNO3 100% Empirical Formula- smallest whole number ratio of elements in a compound HO is empirical formula for H2O2 Empirical Formula Smallest whole number ratio of atoms in a compound C2H6 reduce subscripts CH3 Empirical Formula- smallest whole number ratio of elements in a compound Molecular formula- Actual # of atoms in a molecule as it appears in nature May or may not be the same as empirical For H2O2 HO is empirical formula H2O2 is molecular formula Empirical Formula- smallest whole number ratio of elements in a compound Molecular formula- Actual # of atoms in a molecule as it appears in nature May or may not be the same as empirical For CO2 empirical & molecular are the same Molecular Formula “True Formula” - the actual number of atoms in a compound empirical formula CH3 molecular formula C2H6 Empirical Formula- smallest whole number ratio of elements in a compound Molecular formula- # of atoms in a molecule as it appears in nature May or may not be the same as empirical Empirical? Molecular? Or Both? H2O ? CH4 ? C3H6 ? Empirical Formula- smallest whole number ratio of elements in a compound Molecular formula- Actual # of atoms in a molecule as it appears in nature May or may not be the same as empirical H2O ? Empirical and Molecular CH4 ? Empirical and Molecular C3H6 = Molecular CH2 = Empirical Empirical Formula- smallest whole number ratio of elements in a compound Molecular formula- Actual # of atoms in a compound as it appears in nature May or may not be the same as empirical The mole ratio of the elements in a compound’s Molecular formula is a multiple of the mole ratio of the empirical formula Ex. C6H6 and C2H2 are both multiples of the empirical formula CH Determine the empirical formula of a compound that contains35.98%Al & 64.02%S. Determine the empirical formula of a compound that contains35.98%Al & 64.02%S. Imagine you have 100 g of formula then 35.98 g Al and 64.02 g S Determine the empirical formula of a compound that contains35.98%Al & 64.02%S. Imagine you have 100 g of sample formula then 35.98 g Al and 64.02 g S 35.98 g Al x 1 mole Al = 1.33 moles Al 26.98 g Al Determine the empirical formula of a compound that contains35.98%Al & 64.02%S. Imagine you have 100 g of formula then 35.98 g Al and 64.02 g S 35.98 g Al x 1 mole Al = 1.33 moles Al 26.98 g Al 64.02 g S x 1 mole S = 2.00 moles S 32.07 g S Determine the empirical formula of a compound that contains35.98%Al & 64.02%S. Imagine you have 100 g of formula then 35.98 g Al and 64.02 g S 35.98 g Al x 1 mole Al = 1.33 moles Al 26.98 g Al 64.02 g S x 1 mole S = 2.00 moles S 32.07 g S 1.33 = 1 Al 1.33 2.00 = 1.5 S 1.33 for every 1 Al need 1.5 S Determine the empirical formula of a compound that contains35.98%Al & 64.02%S. Imagine you have 100 g of formula then 35.98 g Al and 64.02 g S 35.98 g Al x 1 mole Al = 1.33 moles Al 26.98 g Al 64.02 g S x 1 mole S = 2.00 moles S 32.06 g S 1.33 = 1 Al 2.00 = 1.5 S for every 1 Al need 1.5 S 1.33 1.33 But can’t be decimal has to be whole number therefore 2 Al and 3 S Al2S3 Determine the empirical formula of a compound that contains 10.89%Mg, 31.77%Cl & 57.34%O? Determine the empirical formula of a compound that contains 10.89%Mg, 31.77%Cl & 57.34%O? If 100 g then 10.89 g Mg x 1 mole Mg = .4480 mole Mg 24.31 g Mg Determine the empirical formula of a compound that contains 10.89%Mg, 31.77%Cl & 57.34%O? If 100 g then 10.89 g Mg x 1 mole Mg = .4480 mole Mg 24.31 g Mg 31.77 g Cl x 1 mole Cl = .8962 mole Cl 35.45 g Cl Determine the empirical formula of a compound that contains 10.89%Mg, 31.77%Cl & 57.34%O? If 100 g then 10.89 g Mg x 1 mole Mg = .4480 mole Mg 24.31 g Mg 31.77 g Cl x 1 mole Cl = .8962 mole Cl 35.45 g Cl 57.34 g O x 1 mole O = 3.584 mole O 16.00 g O Determine the empirical formula of a compound that contains 10.89%Mg, 31.77%Cl & 57.34%O? If 100 g then 10.89 g Mg x 1 mole Mg = .4480 mole Mg 24.31 g Mg 31.77 g Cl x 1 mole Cl = .8962 mole Cl 35.45 g Cl 57.34 g O x 1 mole O = 3.584 mole O 16.00 g O .4480 = 1.00 Mg .8962 = 2.00 Cl 3.584 = 8.00 O .4480 .4480 .4480 Determine the empirical formula of a compound that contains 10.89%Mg, 31.77%Cl & 57.34%O? If 100 g then 10.89 g Mg x 1 mole Mg = .4480 mole Mg 24.31 g Mg 31.77 g Cl x 1 mole Cl = .8962 mole Cl 35.45 g Cl 57.34 g O x 1 mole O = 3.584 mole O 16.00 g O .4480 = 1.00 Mg .8962 = 2.00 Cl 3.584 = 8.00 O .4480 .4480 .4480 MgCl2O8 Molecular Formula 1. Determine empirical formula mass. 2. Divide molecular mass by empirical mass. 3. Multiply each subscript by the answer from step 2 Step 2 MF mass n EF mass Step 3 EF n Calculate molecular formula of compound whose molar mass is 60.0g & the empirical formula is CH4N. Calculate molecular formula of compound whose molar mass is 60.0g & the empirical formula is CH4N. Molar mass of Molecular Formula is 60.0 g Molar Mass of Empirical Formula is CH4N = 12.01 + 4(1.01) + 14.01g = 30.06 g Calculate molecular formula of compound whose molar mass is 60.0g & the empirical formula is CH4N. Molar mass of Molecular Formula is 60.0 g Molar Mass of Empirical Formula is CH4N = 12.01 + 4(1.01) + 14.01g = 30.06 g MF mass = n EF mass (EF)n Calculate molecular formula of compound whose molar mass is 60.0g & the empirical formula is CH4N. Molar mass of Molecular Formula is 60.0 g Molar Mass of Empirical Formula is CH4N = 12.01 + 4(1.01) + 14.01g = 30.06 g MF mass = n EF mass 60.0 g = 2 30.06 mass (EF)n (CH4N)2 = C2H8N2 A compound is composed of 40.68% C, 5.08% H & 54.24%O. It has a molecular mass of 118.1g/mol. Find the empirical & molecular formula. A compound is composed of 40.68% C, 5.08% H & 54.24%O. It has a molecular mass of 118.1g/mol. Find the empirical & molecular formula. 40.68 g C x 1 mole C = 3.387 mole C 12.01 g C 5.08 g H x 1 mole H = 5.03 mole H 1.01 g H 54.24 g O x 1 mole O = 3.39 mole O 16.00 g O A compound is composed of 40.68% C, 5.08% H & 54.24%O. It has a molecular mass of 118.1g/mol. Find the empirical & molecular formula. 40.68 g C x 1 mole C = 3.387 mole C = 1 mole C 12.01 g C 3.387 5.08 g H x 1 mole H = 5.03 mole H = 1.49 mole H 1.01 g H 3.387 54.24 g O x 1 mole O = 3.390 mole O = 1 mole O 16.00 g O 3.387 A compound is composed of 40.68% C, 5.08% H & 54.24%O. It has a molecular mass of 118.1g/mol. Find the empirical & molecular formula. 40.68 g C x 1 mole C = 3.387 mole C = 1 mole C 12.01 g C 3.387 5.08 g H x 1 mole H = 5.03 mole H = 1.49 mole H 1.01 g H 3.387 54.24 g O x 1 mole O = 3.390 mole O = 1 mole O 16.00 g O 3.387 CH1.5O x 2 to eliminate decimal= C2H3O2 = EF A compound is composed of 40.68% C, 5.08% H & 54.24%O. It has a molecular mass of 118.1g/mol. Find the empirical & molecular formula. 40.68 g C x 1 mole C = 3.387 mole C = 1 mole C 12.01 g C 3.387 5.08 g H x 1 mole H = 5.03 mole H = 1.49 mole H 1.01 g H 3.387 54.24 g O x 1 mole O = 3.390 mole O = 1 mole O 16.00 g O 3.387 CH1.5O x 2 to eliminate decimal= C2H3O2 = EF EF mass of C2H3O = 59.05 g A compound is composed of 40.68% C, 5.08% H & 54.24%O. It has a molecular mass of 118.1g/mol. Find the empirical & molecular formula. 40.68 g C x 1 mole C = 3.387 mole C = 1 mole C 12.01 g C 3.387 5.08 g H x 1 mole H = 5.03 mole H = 1.49 mole H 1.01 g H 3.387 54.24 g O x 1 mole O = 3.390 mole O = 1 mole O 16.00 g O 3.387 CH1.5O x 2 to eliminate decimal= C2H3O2 = EF EF mass of C2H3O = 59.05 g 118.1 g = 2 59.05 g A compound is composed of 40.68% C, 5.08% H & 54.24%O. It has a molecular mass of 118.1g/mol. Find the empirical & molecular formula. 40.68 g C x 1 mole C = 3.387 mole C = 1 mole C 12.01 g C 3.387 5.08 g H x 1 mole H = 5.03 mole H = 1.49 mole H 1.01 g H 3.387 54.24 g O x 1 mole O = 3.390 mole O = 1 mole O 16.00 g O 3.387 CH1.5O x 2 to eliminate decimal= C2H3O2 = EF EF mass of C2H3O2 = 59.05 g 118.1 g = 2 = 2 59.05 g (C2H3O2)2 = C4H6O4 Lab: Title: % Composition of Gum Purpose: To find the % of sugar in gum Materials: Watch, gum, balance Procedure: 1. Get balance from cabinet 2. Mass gum IN WRAPPER 3. Take gum out of wrapper & chew for 15 minutes 4. While chewing gum, mass wrapper 5. After 15 min, put gum back in wrapper & get mass Observations: Mass of gum in wrapper _______ Mass of wrapper __________ Mass of gum after chewing _______ Calculations: 1. Find true mass of gum by subtracting the mass of the wrapper from the chewed & unchewed gum ___________ 2. Subtract unchewed mass from chewed mass (this is the mass of the sugar) Find the % sugar in the gum Conclusions: Write a sentence summing up your data Percentage Composition the percentage by mass of each element in a compound mass of element % composition 100 total mass Percentage Composition Find %Cu = %S = the % composition of Cu2S. 127.10 g Cu 159.17 g Cu2S 32.07 g S 159.17 g Cu2S 100 = 79.852% Cu 100 = 20.15% S Percentage Composition Find the percentage composition of a sample that is 28 g Fe and 8.0 g O. %Fe = %O = 28 g 36 g 8.0 g 36 g 100 = 78% Fe 100 = 22% O Percentage Composition How many grams of copper are in a 38.0-gram sample of Cu2S? Cu2S is 79.852% Cu (38.0 g Cu2S)(0.79852) = 30.3 g Cu Percentage Composition Find the mass percentage of water in calcium chloride dihydrate, CaCl2•2H2O? %H2O = 36.04 g 147.02 g 100 = 24.51% H2O Empirical Formula Smallest whole number ratio of atoms in a compound C2H6 reduce subscripts CH3 Empirical Formula 1. Find mass (or %) of each element. 2. Find moles of each element. 3. Divide moles by the smallest # to find subscripts. 4. When necessary, multiply subscripts by 2, 3, or 4 to get whole #’s. Empirical Formula Find the empirical formula for a sample of 25.9% N and 74.1% O. 25.9 g 1 mol = 1.85 mol N 1.85 mol 14.01 g =1N 74.1 g 1 mol = 4.63 mol O 16.00 g 1.85 mol = 2.5 O Empirical Formula N1O2.5 Need to make the subscripts whole numbers multiply by 2 N2O5 Molecular Formula “True Formula” - the actual number of atoms in a compound empirical formula CH3 ? molecular formula C2H6 Molecular Formula 1. Find the empirical formula. 2. Find the empirical formula mass. 3. Divide the molecular mass by the empirical mass. 4. Multiply each subscript by the answer from step 3. MF mass n EF mass EF n Molecular Formula The empirical formula for ethylene is CH2. Find the molecular formula if the molecular mass is 28.1 g/mol? empirical mass = 14.03 g/mol 28.1 g/mol 14.03 g/mol = 2.00 (CH2)2 C2H4 To find empirical formula: 1. Change % to g 2. Change g to mol 3. Find ratio of moles 4. Ratios become subscripts in formula Tricks- if molar ratio is: – .5 X by 2 – .3 or .6 X 3 – .25 or .75 X 4 – .2 or lower, round down – .8 or above round up