Chapter 7 7.4 Name and draw structures of the following complexes?
advertisement
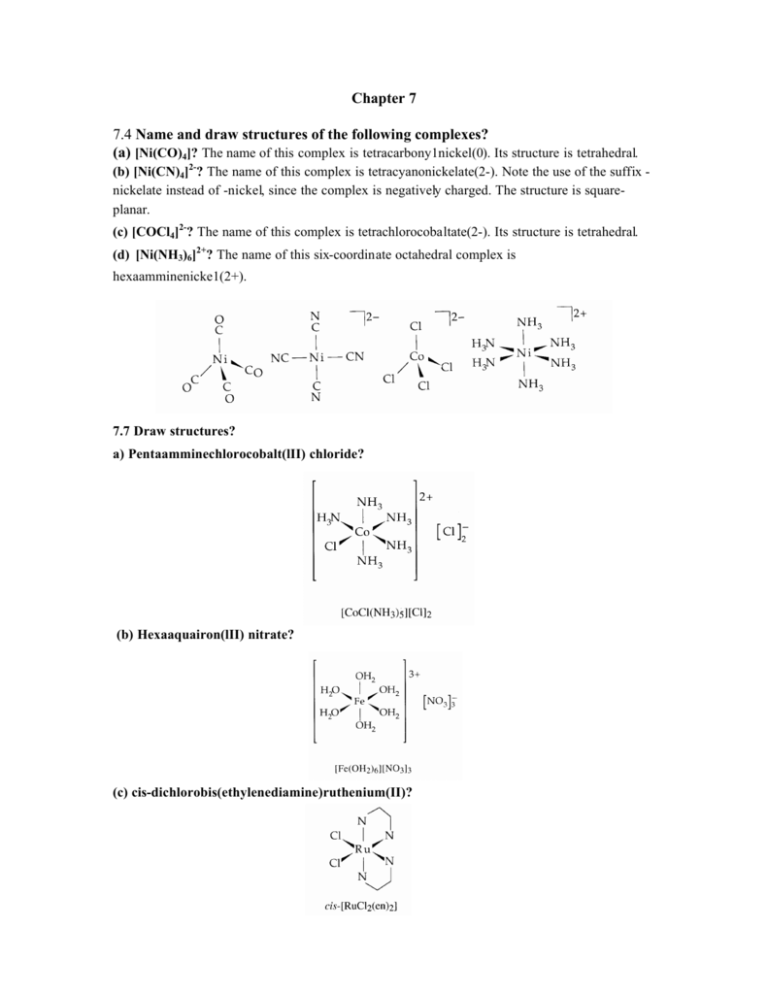
Chapter 7 7.4 Name and draw structures of the following complexes? (a) [Ni(CO)4]? The name of this complex is tetracarbony1nickel(0). Its structure is tetrahedral. (b) [Ni(CN)4]2-? The name of this complex is tetracyanonickelate(2-). Note the use of the suffix nickelate instead of -nickel, since the complex is negatively charged. The structure is squareplanar. (c) [COCl4]2-? The name of this complex is tetrachlorocobaltate(2-). Its structure is tetrahedral. (d) [Ni(NH3)6]2+? The name of this six-coordinate octahedral complex is hexaamminenicke1(2+). 7.7 Draw structures? a) Pentaamminechlorocobalt(lII) chloride? (b) Hexaaquairon(lII) nitrate? (c) cis-dichlorobis(ethylenediamine)ruthenium(II)? (d) µ-Hydroxobis(pentaamminechromium(III)) chloride? 7.8 Name and draw structures? (a) cis-[CrCl2(NH3)4]+? This complex contains (i) two Cl- ligands = dichloro, (ii) four NH3 ligands = tetraammine (not tetraamine or tetrammine), and (iii) Cr3+ in a complex that is not an anion = chromium(III). Putting (i), (ii), and (iii) together, the name of this complex is cistetraamminedichlorochromium(III). Note that the ligands are named in alphabetical order: tetraammine before dichloro. The structure is shown below. (b) trans-[Cr(NCS)4(NH3)2]-? This complex contains four N-bonded NCS-ligands = tetraisothiocyanato, two NH3 ligands = diammine, and Cr3+ in a complex that is an anion (therefore, "chromate(III)" can be used). The name of this complex is transdiamminetetraisothiocyanatochromate(III). Note the alphabetical ordering of the ligands. The structure is shown below. (c) [Co(ox)(en)2]+? This complex contains (i) one oxalate dianion ligand (C2042-) = oxalato, (ii) two ethylenediamine ligands = bis(ethylenediamine), and (iii) Co3+ in a complex that is not an anion = cobalt(III). Therefore, the name of this complex is bis(ethylenediamine)oxalatocobalt(III). The structure is shown above. Note that you do not need to use cis- or trans- for a complex of this composition, since neither of the two types of bidentate ligands in the complex can span two trans positions. 7.9 Draw all possible isomers? (a) Octahedral [RuCl2(NH3)4]? (b) Square-planar [IrH(CO)(PR3)2]? For each compound, cis and trans isomers are possible. (c) Tetrahedral [CoCl3(OH2)]-? (d) Octahedral [IrCl3(PEt 3)3]? There is only one isomer for (c), but two isomers, one fac (facial) and one mer (meridianal), for (d), as shown below. (e) Octahedral [CoCl2(NH3)2(en)]+? There are three isomers. In one, the two Cl- ligands are trans to one another. In the other two, the two CIligands are cis to one another. The three isomers are shown below (the 1+ charge for each of these three isomers is not shown). 7.10 Draw and name iridium complexes? Structures of the three complexes are shown below. You should recognize the logic behind putting the two bulky triphenylphosphine ligands opposite one another in the two trigonalbipyramidal complexes. The names of the three complexes, from left to right, are carbonyl( chloro)bis(triphenylphosphine)iridium(I), dicarbonyl(chloro)bis(triphenylphosphine)iridium(I), and dicarbonyl(hydrido)bis(triphenylphosphine)iridium(I). Note the alphabetical ordering of the ligands, such as dicarbonyl before chloro. 7.23 Assign the colors pink, yellow, and blue to cobalt(lI) complexes? The colors of metal complexes are frequently due to ligand-field transitions involving electron promotion from one subset of d orbitals to another (e.g. from t2g to e g for octahedral complexes or from e to t2 for tetrahedral complexes). Of the three complexes given, the lowest energy transition probably occurs for [COCl4]2-, because it is tetrahedral (∆T = (4/9)(∆O)) and because Cl- is a weak field ligand. This complex is blue, since a solution of it will absorb low energy red light and reflect the complement of red, which is blue. Of the two complexes that are left, [Co(NH3)6]2+ probably has a higher energy transition than [CO(OH2)6]2+, since NH3 is a stronger-field ligand than H2O (see Table 7.3). The complex [Co(NH3)6]2+ is yellow because only a small amount of visible light, at the blue end of the spectrum, is absorbed by a solution of this complex. By default, you should conclude that [Co(OH2)6]2+ is pink.