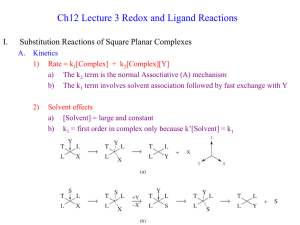
APPLIED INORGANIC CHEMISTRY (CHEM261) Transition Metal Chemistry The elements in the periodic table are often divided into four categories: (1) main group elements, (2) transition metals, (3) lanthanides, and (4) actinides. Main-group elements S-Block Main-group elements P-Block Transition metals Lanthanide Actinides There is some controversy about the classification of the elements on the boundary between the main group and transition-metal elements on the right side of the table. The elements in question are zinc (Zn), cadmium (Cd), and mercury (Hg). 1 Transition metals Main-group elements P-Block The Electron Configuration of Transition-Metal Ions The relationship between the electron configurations of transition-metal elements and their ions is complex. Example 2+ 3+ Consider the chemistry of cobalt which forms complexes that contain either Co or Co ions. Co : 2+ : 3+ : Co Co In general, electrons are removed from the valence shell s orbitals before they are removed from valence d orbitals when transition metals are ionized. How do we determine the electronic configuration of the central metal ion in any complex? Try to recognise all the entities making up the complex and knowing whether the ligands are neutral or anionic, you can determine the oxidation state of the metal ion. A simple procedure exists for the M(II) case. 22 23 24 25 26 27 28 29 Ti V Cr Mn Fe Co Ni Cu Evaluating the oxidation state [CoCl(NO2)(NH3)4]+ 2 Oxidation States and their Relative Stabilities Why do these elements exhibit a variety of oxidation states? The most prevalent oxidation numbers are shown in bold font. Sc +3 Ti +1 +2 +3 +4 V +1 +2 +3 +4 +5 Cr +1 +2 +3 +4 +5 +6 Mn +1 +2 +3 +4 +5 +6 Fe +1 +2 +3 +4 +5 +6 Co +1 +2 +3 +4 +5 Ni +1 +2 +3 +4 Cu +1 +2 +3 Zn +7 +2 An increase in the number of oxidation states from Sc to Mn. All seven oxidation states are exhibited by Mn. Why is there a decrease in the number of oxidation states from Mn to Zn? The stability of higher oxidation states decreases in moving from Sc to Zn. Mn(VII) and Fe(VI) are powerful oxidizing agents and the higher oxidation states of Co, Ni and Zn are unknown. The relative stability of +2 state with respect to higher oxidation states, particularly +3 state increases in moving from left to right. This is justifiable since it will be increasingly difficult to remove the third electron from the d-orbital. 3 Properties Summary of Physical Properties 1. Have large charge/radius ratio 2. Are hard and have high densities 3. Have high melting and boiling points 4. Form compounds which are often paramagnetic 5. Show variable oxidation states 6. Form coloured ions and compounds 7. Form compounds with profound catalytic activity 8. Form stable complexes Coordination Chemistry Definitions Coordination compound (coordination complex) - contains a central metal atom or ion surrounded by a number of oppositely charged ions or neutral molecules (possessing lone pairs of electrons) which are known as ligands. Metal chelates - ligand capable of forming more than one bond with the central metal atom or ion, producing ring structures. Ring forming groups are described as chelating agents or polydentate ligands. Coordination number - total number of sites occupied by ligands around the central metal atom. 4 Note: a bidentate ligand uses two sites, a tridentate three sites etc. Molecular formula [Zn(CN)4] [PtCl6] 2- 22+ [Ni(NH3)6] Lewis Base/Ligand CN - Lewis Acid Donor Atom Coordination No. 2+ C 4 4+ Cl 6 2+ N 6 Zn Cl - Pt NH3 Ni Example of Ligands Mono-dentate Multidentate Ligands Chelating ligands bonded to metal – rings – chelate rings - any number of atoms in the ring, most common – five or six atoms, including metal. 5 Nomenclature Common monodentate ligands 6 Common multidentate (chelating) ligands 7 8 Examples: tetrachloroferrate(III), [FeCl4] - dicyanoaurate(I), ______________ 9 Exercise 1 Name the following coordination complexes: (i) Cr(NH3)Cl3 (ii) Pt(en)Cl2 (iii) [Pt(ox)2] 2- (iv) [Cr(H2O)5Br] (v) 2+ [Cu(NH2CH2CH2NH2)Cl4]2- (vi) [Fe(OH)4] Exercise 2 Give the structures of the following coordination complexes: (i) Tris(acetylacetanato)iron(III) (ii) Hexabromoplatinate(2-) (iii) Potassium diamminetetrabromocobaltate(III) (iv) Tris(ethylenediamine)copper(II) sulphate (v) Hexacarbonylmanganese(I) perchlorate (vi) Ammonium tetrachlororuthenate(1-) Isomerism Coordination Numbers and Geometries 10 Isomers occur primarily in coordination numbers 4 and 6. Isomers obtained by the arrangement of ligands in space and also depend on the ligands themselves. Ionization isomers Compounds with the same formula, but gives different ions in solution. Difference is which ion is present as the ligand and which is present to balance the overall charge. [Co(NH3)4(H2O)Cl]Br2 and [Co(NH3)5SO4]NO3 and [Co(NH3)4(NO2)Cl]Cl and [Co(NH3)4Br2]Cl∙H2O Coordination isomers In compounds, both cation and anion are complex, the distribution of ligands can vary, giving rise to isomers. 3+ [Co(NH3)6] -3 [Cr(CN)6] and -3 [Co(CN)6] +3 [Cr(NH3)6] Other examples [Co(en)3][Cr(CN)6] and [Pt(NH3)4][PtCl6] and Pt(II) [Cr(en)3][Co(CN)6] Pt(IV) Linkage isomers Some ligands can bond to the metal through different atoms, e.g. nitro and nitito, N or O coordination possible. (yellow) (red) 11 Geometric isomers Formula is the same but the arrangement in 3-D space is different e.g. square planar molecules give cis and trans isomers. Also possible for 6- or hexacoordinate species For hexacoordinate complexes of the formula, ML3L3’ where L and L’ are monodentate ligands, also have two isomeric forms called fac- and mer- (facial and meridional). Fac has 3 identical ligands on one triangular face; mer have 3 identical ligands in a plane bisecting the molecule. Stereoisomers Enantiomers (non-superimposable mirror images) 12 Complex Stabilities In aqueous solution a comparison of metal complexes and their affinity for the H2O molecule as a competing ligand has been studied. Here are some general observations: (i) For a given metal and ligand, complexes with the metal oxidation state +3 are more stable than +2. (ii) Stabilities of complexes of the first row of transition metals vary in reverse of their cationic radii (in II II II II II II general): Mn < Fe < Co < Ni > Cu > Zn (iii) Hard and soft Lewis acid-base theory: in general, acids are identified as hard or soft by the thermodynamic stability of the complexes they form as shown: Hard acids bond in the order: R3P « R3N, R2S « R2O Soft acids bond in the order: R2O « R2S, R3N « R3P For example, the Lewis acid phenol forms a more stable complex by hydrogen bonding to (C2H5)2O than (C2H5)2S. By contrast, the Lewis acid I2Forms a more stable complex with (C2H5)2S than (C2H5)2O; conclude that phenol is hard whereas I2 is soft. Similar behaviour with bases. (iv) Chelate effect - Effect is the additional stability of a complex containing a chelating ligand, relative to that of a complex containing monodentate ligands with the same type and number of donors as in the chelate. 2+ 2+ H 2O H 2O H 2O H 2O NH3 NH2 Cu Cu CH2 CH2 H 2O H2O NH3 NH2 H2O H2O 2+ Cu(H2O)4(NH3)2] 2+ + en = [Cu(H2O)4(en)] + 2 NH3 When ammonia molecule dissociates - swept off in solution and the probability of returning is remote. When one amine group of en dissociates from complex ligand retained by end still attached so the nitrogen atom cannot move away – swings back and attach to metal again. Therefore the complex has a smaller probability of dissociating. 13