Information: Units
advertisement

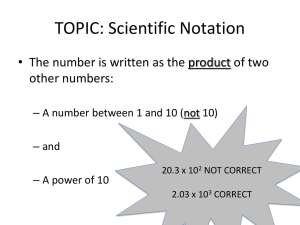
Physics Fix 1 & 2 Name: ______________________________ Date: _____________ Hour: _____ Information: Units The following tables contain common metric (SI) units and their prefixes. Table 1: metric base units Quantity Length Mass Time Temperature Volume Amount of substance Unit meter kilogram second Kelvin Liter mole Table 2: prefixes for metric base units. Prefix Symbol Mega M Kilo k Deci d Centi c Milli m Micro Nano n Pico p Unit Symbol m kg s K L mol Meaning in Words million thousand tenth hundredth thousandth millionth billionth trillionth Meaning in Numbers 1,000,000 1,000 0.1 0.01 0.001 0.000001 0.000000001 0.000000000001 Note the following examples: “Mega” means million so “Megagram” (Mg) means one million grams NOT one millionth of a gram. One millionth of a gram would be represented by the microgram (g). It takes one million micrograms to equal one gram and it takes one million grams to equal one Megagram. One cm is equal to 0.01 m because one cm is “one hundredth of a meter” and 0.01 m is the expression for “one hundredth of a meter” Critical Thinking Questions 1. “Milli” means “thousandth” so a milliliter (symbol: mL) is one thousandth of a Liter. Therefore, it takes _____________ mL to make one L 2. “Kilo” means “thousand” so a kiloliter contains ____________ Liters? how many? 3. How many milligrams are there in one kilogram? 4. How many meters are in 21.5 km? 5. Is it possible to answer this question: How many mg are in one km? Explain. 6. Which is larger one Mm or one mm? Information: Scientific Notation “Scientific notation” is used to make very large or very small numbers easier to handle. For example the number 45,000,000,000,000,000 can be written as “4.5 x 1016 ”. The “16” tells you that there are sixteen decimal places between the right side of the four and the end of the number. Another example: 2.641 x 1012 = 2,641,000,000,000 the “12” tells you that there are 12 decimal places between the right side of the 2 and the end of the number. Very small numbers are written with negative exponents. For example, 0.00000000000000378 can be written as 3.78 x 10-15. The “-15” tells you that there are 15 decimal places between the right side of the 3 and the end of the number. Another example: 7.45 x 10-8 = 0.0000000745 the “-8” tells you that there are 8 decimal places between the right side of the 7 and the end of the number. In both very large and very small numbers, the exponent tells you how many decimal points are between the right side of the first digit and the end of the number. If the exponent is positive, the decimal places are to the right of the number. If the exponent is negative, the decimal places are to the left of the number. Critical Thinking Questions 7. Two of the following six numbers are written incorrectly. Circle the two that are incorrect. a) 3.57 x 10-8 104 b) 4.23 x 10-2 c) 75.3 x 102 d) 2.92 x 109 What do you think is wrong about the two numbers you circled? 8. Write the following numbers in scientific notation: e) 0.000354 x 104 f) 9.1 x a) 25,310,000,000,000,000 = _____________ b) 0.000000003018 = ____________ 9. Write the following scientific numbers in regular notation: a) 8.41 x 10-7 = ________________ b) 3.215 x 108 = _____________________ Information: Multiplying and Dividing Using Scientific Notation When you multiply two numbers in scientific notation, you must add their exponents. Here are two examples. Make sure you understand each step: (4.5x1012) x (3.2x1036) = (4.5)(3.2) x 1012+36 = 14.4x1048 1.44x1049 (5.9x109) x (6.3x10-5) = (5.9)(6.3) x 109+(-5) = 37.17x104 3.717x105 When you divide two numbers, you must subtract denominator’s exponent from the numerator’s exponent. Here are two examples. Make sure you understand each step: 2.8 x1014 2.8 x10147 0.875 x107 8.75 x106 7 3.2 x10 3.2 5.7 x1019 5.7 x1019 ( 9 ) 1.84 x1019 9 1.84 x10 28 9 3.1 3.1x10 Critical Thinking Questions 11. Solve the following problems. a) (4.6x1034)(7.9x10-21) = b) (1.24x1012)(3.31x1020) = 12. Solve the following problems. a) 8.4 x10 5 4.1x1017 b) 5.4 x10 32 7.3 x1014 Information: Adding and Subtracting Using Scientific Notation Whenever you add or subtract two numbers in scientific notation, you must make sure that they have the same exponents. Your answer will them have the same exponent as the numbers you add or subtract. Here are some examples. Make sure you understand each step: 4.2x106 + 3.1x105 make exponents the same, either a 5 or 6 42x105 + 3.1x105 = 45.1x105 = 4.51x106 7.3x10-7 - 2.0x10-8 make exponents the same, either -7 or -8 73x10-8 – 2.0x10-8 = 71x10-8 = 7.1x10-7 Critical Thinking Questions 13. Solve the following problems. a) 4.25x1013 + 2.10x1014 = b) 6.4x10-18 – 3x10-19 = c) 3.1x10-34 + 2.2x10-33 = CONVERTING UNITS Information: Dimensional Analysis “Dimensional Analysis” is a big scary term that doesn’t really need to be scary. It’s simple. The basis for dimensional analysis is this: if you multiply something by 1 you do not change its value! Pretty easy, eh? Here’s an example: 1 3 3 2 3 6 Notice that the value of ½ didn’t really change because 3/3 is the same as 1. Again, in mathematics, multiplying by 1 doesn’t change the real value of anything. 100 cm 3 3 100 cm is a fraction t hat behaves just like because 100cm 1 meter! Therefore, neither nor 1 meter 3 3 1 meter will change the real value of a number. Here’s an example problem of a conversion: Convert 3.75 cm into meters. All you need to do is multiply by a fraction. Always begin by putting the number you are given in a fraction over 1. Find a fraction that contains both units that you are working with. Here we have cm and m. Notice that 1 m and 100 cm equal each other. THIS IS A MUST. You could also have “0.01 m” and “1 cm” because 0.01 m = 1 cm. 3.75 cm 1m 3.75 cm 1m 3.75 cm 1m 0.0375 m 1 100 cm 1 100 cm 1 100 cm We put cm on the bottom here so that it cancels here. m is the only unit left and it’s the unit we want Notice in the above example that cm was on the bottom in the conversion factor fraction. This is very important. “Tops and bottoms cancel each other.” We need cm on the bottom so that it cancels out the one on the top! Critical Thinking Questions 1. If you were converting 42 grams into kilograms, which fraction would you use as a converting factor? A) 1000 g 1 kg B) 1000 kg 1g C) 1 kg 1000 g D) 1g 1000 kg Explain your reasoning: 2. How many meters are in 32.5 kilometers? (You are converting km to m.) The problem is started for you: 32.5 km 1 3. How many L are there in 32.5 L? Information: Non-base unit non-base unit So far we have been converting a prefixed unit into a base unit or vice versa. It gets a little more complex when we want to convert a prefixed unit into another prefixed unit. Whenever such is the case, convert to the base unit first and then finish the problem. For example, if you needed to convert centimeters into kilometers, first convert to the base unit—meters. Then convert meters into kilometers. Critical Thinking Questions 4. How many cm are there in 40 km? Let’s break it into two steps… a) First, convert to the base unit, which for this problem is meters. Fill in the blanks. 40 km 1 m ______________________ km km is on the bottom to cancel out the other km, which is on the top. m and km are chosen because we are converting from km to m b) Now convert your answer to part a (which is in meters) into centimeters. m 1 5. How many kL are there in 34,500 mL? km m a) First, convert mL to L. b) Now convert your answer to part a (in L) to kL. 6. How many m are there in 0.0035 km? Information: Quantities containing two units at once It gets a bit more complicated if we have to convert a quantity containing two units. For example, speed has two units. “Miles per hour” contains two units. “Meters per second” contains two units. When you need to do a conversion on such a quantity, do one unit at a time. Here’s an example. Convert 50 km/hr to m/s. hr is on the top here so that it cancels with the hr on the bottom. “Tops and bottoms cancel.” 50 km 1000 m 1 hr 1 min 50 km 1000 m 1 hr 1 min 13.89 m/s 1 hr 1 km 60 min 60 s 1 hr 1km 60 min 60 s First we converted km then we’ll work on the hr. Critical Thinking Questions 7. Convert 25 m/s to km/hr. 8. The speed of sound is approximately 340 m/s. How many km/hr is that? 9. The maximum highway speed in Michigan is 70 miles/hr. How many km/hr is this? (Note: 1 mile is equal to 1609 m.) 10. The flow of water in our kitchen tap is 3.2 L/min. How many mL/s is this?