atom
advertisement
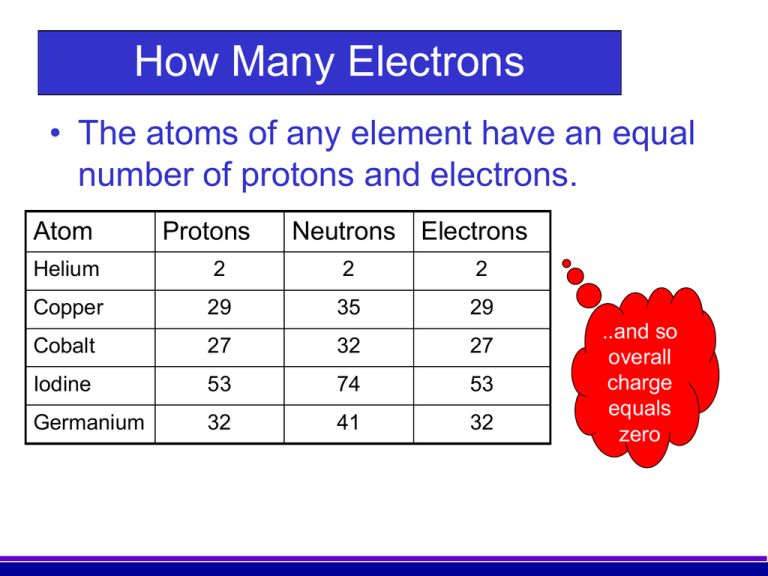
How Many Electrons • The atoms of any element have an equal number of protons and electrons. Atom Protons Neutrons Electrons Helium 2 2 2 Copper 29 35 29 Cobalt 27 32 27 Iodine 53 74 53 Germanium 32 41 32 ..and so overall charge equals zero Activity How Many Electrons • Fill in the blank columns Atom Protons Neutrons Electrons Boron 5 6 Potassium 19 20 Chromium 24 28 Mercury 80 121 Argon 18 22 Atomic number Mass Number 5 19 5 19 11 39 24 80 18 24 80 18 52 201 40 Note – atomic number is defined as the number of protons rather than electrons because atoms can lose (or gain) electrons but do not normally lose protons Activity Drag the statements at the top onto the correct side of the table Activity Drag the statements at the top onto the correct side of the table How Are Electrons Arranged? • Electrons are not evenly spread. • The exist in layers known as shells. • The arrangement of electrons in these shells is often called the electron configuration. 1st Shell 2nd Shell 3rd Shell 4th Shell How Many Electrons per Shell? • Each shell has a maximum number of electrons that it can hold. The maximum 1st Shell: 2 electrons 2nd Shell: 8 electrons 3rd Shell: 8 electrons Working Out Electron Arrangements 1. How many electrons do the element atoms have? (This will equal the atomic number). 2. Draw them into the shells working outwards until you have used them all up. Drawing neat diagrams helps you keep track! 1st Shell: Fills this first 2nd Shell: Fill this next The Electrons in Carbon The Electrons in Neon The Electrons in Silicon Electrons in Phosphorus The Electrons in Argon The Electrons in Sodium The Electrons in Fluorine The Electrons in Aluminium The Electrons in Nitrogen The Electrons in Sulfur The Electrons in Oxygen The Electrons in Chlorine The Electrons in Magnesium Happiness is…………………. A full outer shell Ions and Electron Structures 1. Ions are atoms that have either extra electrons added or electrons removed. e.g. Atoms Protons Electrons Lose 1 electron 1+ ion Gain 1 electron Protons Electrons So in ions the number of electrons no longer equals the number of protons 1- ion Protons Electrons Positive Ions • These are ions formed by the atom losing one or more electrons. Lost 1 e- • • • + They are called cations. This is because during electrolysis (Or It is nearly they move towards the cathode. Ions usually always they are Lost because “pussytive”!). have 2 e2+ metal OUTER atoms that on the ions is equal to theelectron The charge number of lose electrons that the atom has lost. shells that Lost 3 e electrons 3+ are either completely In equations the charge is usually shown above 2+or).else full and to the right of the symbol. (E.g. Mg empty The Electrons in a Sodium Ion In the sodium atom Atomic number = number of protons Number of electrons = 11 Na 23 = 11 Na 11 Na+ Bye! Electron lost Electron arrangement: 2.8.1 (Incomplete Shell) Electron arrangement: 2.8 (Full Shells) The Electrons in a Magnesium Ion In the magnesium atom Atomic number = number of protons Number of electrons = 12 24 = 12 Mg 12 Bye! Mg2+ Mg Bye! Electron arrangement = 2.8.2 (Incomplete shell) 2 electrons lost Electron arrangement 2.8 (Full Shells) Activity The Lithium Ion •How many electrons? 3 •How many electrons in the first shell? 2 •How many electrons in the second shell? 1 What electron arrangement? 7 Li 3 2.1 1st Shell = 2: full 2nd Shell = 1: not full How many electrons to lose? 1 Li Li+ New electron arrangement? Include a diagram 2.(0) Bye! Activity The Boron Ion •How many electrons? 5 •How many electrons in the first shell? 2 •How many electrons in the second shell? 3 What electron arrangement? 11 B 5 2.3 1st Shell = 2: full 2nd Shell = 3: not full How many electrons to lose? New electron arrangement? Bye! Bye! 3 2.(0) BB3+ Bye! Negative Ions. • These are ions formed by the atom gaining one or more electrons. Gain 1 e • They are called anions. This is because during It is nearly Ions usually electrolysis they move towards the anode. always have non-metal OUTER The charge number of atoms that on the ions is equal to theelectron gain electrons that the atom has gained.shells that Gain 3 eelectrons 3are either completely In equations the charge is usually shown above 2- or and to the right of the symbol. (E.g.. Ofull ). else empty Gain 2 e • • - 2- The Electrons in a Sulphide Ion. In the sulphur atom Atomic number = number of protons Number of electrons = 16 32 = 16 S2- S 2 electrons gained Electron arrangement: 2.8.6 (incomplete shell) Electron arrangement 2.8.8 (Full shells) S 16 The Electrons in a Fluoride Ion. In the fluorine atom Atomic number = number of protons Number of electrons = 9 19 =9 F F2- F 1 electron gained Electron arrangement: 2.8.7 (incomplete shell) Electron arrangement 2.8.8 (Full shells) 9 Activity The Oxide Ion 8 •How many electrons? •How many electrons in the first shell? 2 •How many electrons in the second shell? 6 What electron arrangement? 16 O 8 2.6 1st Shell = 2: full 2nd Shell = 6: not full How many electrons to gain? 2 OO2- New electron arrangement? 2.8 Drag the words at the top to their correct places in the sentences. Word check 1. Which of the following is not a subatomic particle? A. B. C. D. Proton. Isotope. Neutron. Electron. 2. The element Cobalt has a relative atomic mass of 59 and an atomic number of 27. Which of these is a true statement about each neutral cobalt atom? A. B. C. D. It contains 59 neutrons. It contains 27 electrons. It contains 32 protons. It contains equal numbers of neutrons and electrons. 3. The Periodic Table displays iron as shown below. This indicates that Fe atoms: 56 A. B. C. D. Fe contain 56 neutrons. 26 contain 30 electrons. contain 26 protons. contains more protons than neutrons. 4. Bromine consists of a mixture of two isotopes: Bromine-79 and Bromine-81 • Which of the following is true: 80 Br A. B. C. D. 35 Both isotopes contain 35 protons. Bromine 79 contains 46 neutrons. Bromine 81 contains 44 neutrons. Bromine-81 is more reactive than bromine79. 5. Natural boron consists of approx. 20% boron-10 and 80% boron-11. • What will the relative atomic mass of natural boron will be? ? A. B. C. D. 10.0 11.0 10.5 10.8 B 5 6. Which answer best describes the shell arrangement of the electrons in a sodium atom? 23 Na 11 A. B. C. D. 2,8,1. 2,2,7. 2,8,8,3. 2,8,8,1. 7. Which answer best describes the shell arrangement of the electrons in an oxygen atom? 16 O A. B. C. D. 2,8,6. 2,8,8. 2,8. 2,6. 8 8. Which of these elements will have electron shells that are either full or empty (i.e. not partially full)? A 14 N B 7 C 40 Ca 20 40 Ar 18 D 27 Al 13 9. Which answer best describes the shell arrangement of the electrons in an oxide ion O2-? 16 O A. B. C. D. 2,8,6. 2,8,8. 2,8. 2,6. 8




