boiling point - My Teacher Pages
advertisement

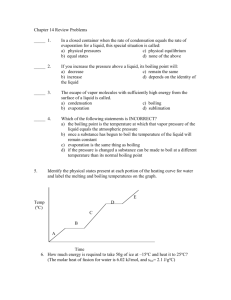
Liquid-Vapor Equilibrium (Intermolecular Forces and Liquids and Solids) Green/Damji: 17.1 Chang: Chapter 11 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Write an equation for the equilibrium between liquid water and its vapor: Is evaporation an endothermic or exothermic process? Condensation Focus on Evaporation and Condensation Evaporation Least Order Greatest Order 11.8 Before Evaporation At Equilibrium 11.8 The equilibrium vapor pressure is the vapor pressure measured when a dynamic equilibrium exists between condensation and evaporation H2O (l) H2O (g) Dynamic Equilibrium Rate of Rate of = evaporation condensation 11.8 Factors affecting the vapor pressure • SURFACE AREA increasing surface area only means equilibrium is reached faster • TEMPERATURE What is the name of this diagram? Why is it important? T2 > T1 11.8 Vapor Pressure Versus Temperature Patterns? Why? 11.8 Enthalpy of vaporization (DHvap) is the energy required to vaporize 1 mole of a liquid at its boiling point. Enthalpy of vaporization : physical process (phase change) as ______________________: chemical reaction 11.8 The relationship between vapor pressure and temperature can be expressed mathematically – NOT req’d by IB Clausius-Clapeyron Equation DHvap ln P = +C RT P = (equilibrium) vapor pressure T = temperature (K) R = gas constant (8.314 J/K•mol) C = integration constant Vapor Pressure Versus Temperature 11.8 Heating Curve Why are there plateaus on this graph? 11.8 The boiling point is the temperature at which the (equilibrium) vapor pressure of a liquid is equal to the external pressure. The normal boiling point is the temperature at which a liquid boils when the external pressure is 1 atm. 11.8 What are the three primary intermolecular forces? List in order of increasing strength. The boiling point and enthalpy of vaporization are influenced by intermolecular forces… Compound methane CH Enthalpy of vaporization in kJ mol-1 Boiling Point in K 9.0 109 27.2 248 38.6 352 4 methoxy methane CH OCH 3 3 ethanol CH CH OH 3 2 Intermolecular Forces State the relationship between intermolecular forces and enthalpy of vaporization. State the relationship between intermolecular forces and boiling point. A phase diagram summarizes the conditions at which a substance exists as a solid, liquid, or gas. Phase Diagram of Water 11.9 Effect of Increase in Pressure on the Melting Point of Ice and the Boiling Point of Water 11.9 Textwork 17.1 Read 17.1 on pp 192-193 Address each of the learning objectives on p. 192 by taking notes that directly relate to the objectives. Due on __________________