Surveillance of scrapie (ELISA, WB, IHC)
advertisement

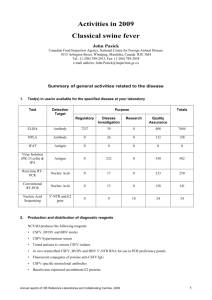
OIE Reference Laboratory Reports Activities in 2012 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Bovine Spongiform Encephalopathy Address of laboratory 3-1-5 Kannondai, Tsukuba, Ibaraki 305-0856 JAPAN Tel.: +81-29-939-7840 Fax: +81-29-939-8332 e-mail address: tyoko@affrc.go.jp website: http://www.naro.affrc.go.jp/org/niah/ Name (including Title) of Head of Laboratory (Responsible Official): Dr. Takafumi Hamaoka Name (including Title and Position) of OIE Reference Expert: Dr. Takashi Yokoyama Date of submission to the OIE Instructions This form should be used by an OIE Reference Laboratory to report activities that took place from January through December of the past year (2012), unless otherwise stated, and must be submitted by the end of January every year. Only those activities that concern the disease (or topic) for which the laboratory is recognised by the OIE should be mentioned. The questionnaire structure follows the Terms of Reference (ToRs) for OIE Reference Laboratories, available at: http://www.oie.int/en/our-scientific-expertise/reference-laboratories/introduction/ Each ToR (blue italicised text) has been placed as a heading covering the group of questions related to it. Please note the red italicised text is given as guidance and should be deleted from your report and substitute with your data. Examples are based on past Annual Reports or have been invented. The questionnaire represents a means of gathering information on activities carried out by OIE Reference Laboratories and making it available to OIE Member Countries and to the OIE Reference Laboratory network. This annual report will remain available for consultation on the OIE web site: (http://www.oie.int/en/our-scientific-expertise/reference-laboratories/annual-reports/): Annual reports of OIE Reference Centres, 2012 1 OIE RL for « Bovine Spongiform Encephalopathy » – « Takashi Yokoyama » – « Japan » Test recommended by the OIE 2. Total number of test performed last year Direct diagnostic tests Nationally Internationally Confirmatory test for BSE (WB, IHC) 0 0 Surveillance of scrapie (ELISA, WB, IHC) 154 0 Surveillance of CWD (WB) 21 0 Did your laboratory produce or store imported standard reference reagents officially recognised by the OIE or other international bodies? Yes 3. No Did your laboratory supply standard reference reagents to OIE Member Countries? Yes Type of reagent available Control BSE positive brain samples 4. Related diagnostic test WB, ELISA No Produced/ stored Amount supplied nationally (ml, mg) Amount supplied internationally (ml, mg) Name of recipient OIE Member Countries and of institutions Produced 3(positive) 3g (gx3) QIA, South Korea Did your laboratory produce diagnostic reagents other than the OIE-approved standard reference reagents? Yes 5. No Did your laboratory produce vaccines? Yes 6. Did your laboratory supply vaccines to OIE Member Countries? Yes 7. No Did your laboratory develop new vaccines according to OIE Standards for the designated pathogen or disease? Yes Name of the new test or diagnostic method or vaccine developed PMCA (Protein misfolding cyclic amplification) 2 No Did your laboratory develop new diagnostic methods validated according to OIE Standards for the designated pathogen or disease? Yes 8. No No Description and References (Publication, website, etc.) Okada H et al., (2012). rion in saliva of bovine spongiform encephalopathy-infected cattle. Emerg Infect Dis. 18: 2091-2. Annual reports of OIE Reference Centres, 2012 OIE RL for « Bovine Spongiform Encephalopathy » – « Takashi Yokoyama » – « Japan » 9. Did your laboratory carry out diagnostic testing for other OIE Member Countries? Yes 10. No Did your laboratory provide expert advice in technical consultancies on the request of an OIE Member Country? Yes No Name of the OIE Member Country receiving a technical consultancy Purpose How the advice was provided National Institute of Animal Health, Taiwan BSE surveillance strategy In loco Science Academia, Taiwan Inactivation of prion In loco QIA, South Korea Diagnosis of TSE In loco 11. Did your laboratory participate in international scientific studies in collaboration with OIE Member Countries other than the own? Yes No Title of the study Duration Purpose of the study Establishment of CWD-prion susceptible cell lines 1 year To establish the in vitro assay system for CWD prions 12. Partners (Institutions) OIE Member Countries involved other than your country QIA, Anayng South Korea Did your Laboratory collect epizootiological data relevant to international disease control? Yes 13. Did your laboratory disseminate epizootiological data that had been processed and analysed? Yes 14. No No What method of dissemination of information is most often used by your laboratory? (Indicate in the appropriate box the number by category) a) Articles published in peer-reviewed journals: ................. 14 b) International conferences: .............................................. 13 c) National conferences: ...................................................... 0 d) Other: ............................................................................... 0 a) Published peer-reviewed journals 1.Okada H, Iwamaru Y, Fukuda S, Yokoyama T, Mohri S. (2012) Detection of disease-associated prion Annual reports of OIE Reference Laboratories, 2012 3 OIE RL for « Bovine Spongiform Encephalopathy » – « Takashi Yokoyama » – « Japan » protein in the optic nerve and the adrenal gland of cattle with bovine spongiform encephalopathy by using highly sensitive immunolabeling procedures. J Histochem Cytochem. 60: 290-300. 2. Fukuda S, Onoe S, Nikaido S, Fujii K, Kageyama S, Iwamaru Y, Imamura M, Masujin K, Matsuura Y, Shimizu Y, Kasai K, Yoshioka M, Murayama Y, Mohri S, Yokoyama T, Okada H. (2012) Neuroanatomical distribution of disease-associated prion protein in experimental bovine spongiform encephalopathy in cattle after intracerebral inoculation. Jpn J Infect Dis. 65: 37-44. 3. Kasai K, Hirata A, Ohyama T, Nokihara K, Yokoyama T, Mohri S. (2012) Novel assay with fluorescencelabelled PrP peptides for differentiating L-type atypical and classical BSEs, and scrapie. FEBS Lett. 586: 325-9. 4. Masujin K, Miwa R, Okada H, Mohri S, Yokoyama T. (2012) Comparative analysis of Japanese and foreign L-type BSE prions. Prion 6: 89-93. 5. Tang Y, Gielbert A, Jacobs JG, Baron T, Andreoletti O, Yokoyama T, Langeveld JP, Sauer MJ. (2012) All major prion types recognised by a multiplex immunofluorometric assay for disease screening and confirmation in sheep. J Immunol Methods. 380: 30-9. 6. Murayama Y, Imamura M, Masujin K, Shimozaki N, Yoshioka M, Mohri S, Yokoyama T. (2012) Ultrasensitive detection of scrapie prion protein derived from ARQ and AHQ homozygote sheep by interspecies in vitro amplification. Microbiol Immunol. 56:541-7. 7. Kim HJ, Tark DS, Lee YH, Kim MJ, Lee WY, Cho IS, Sohn HJ, Yokoyama T. (2012) Establishment of a Cell Line Persistently Infected with Chronic Wasting Disease Prions. J Vet Med Sci. 74: 1377-1380. 8. Dorj G, Okada H, Miyazawa K, Masujin K, Kimura K, Mohri S, Yokoyama T. (2012) Retrospective Analysis of Sheep Scrapie by Western Blotting with Formalin-Fixed Paraffin-Embedded (FFPE) Tissues. J Vet Med Sci. 74: 1207-1210. 9. Iwamaru Y, Takenouchi T, Murayama Y, Okada H, Imamura M, Shimizu Y, Hashimoto M, Mohri S, Yokoyama T, Kitani H. (2012) Anti-prion activity of Brilliant Blue G. PLoS One. 7:e37896. 10. Takenouchi T, Iwamaru Y, Imamura M, Fukuhara S, Sugama S, Sato M, Mochizuki N, Hashimoto M, Yokoyama T, Mohri S, Kitani H. (2012) Cytochalasin D enhances the accumulation of a protease-resistant form of prion protein in ScN2a cells: involvement of PI3 kinase/Akt signalling pathway. Cell Biol Int. 36: 1223-31. 11. Okada H, Murayama Y, Shimozaki N, Yoshioka M, Masujin K, Imamura M, Iwamaru Y, Matsuura Y, Miyazawa K, Fukuda S, Yokoyama T, Mohri S. (2012) Prion in saliva of bovine spongiform encephalopathy-infected cattle. Emerg Infect Dis. 18:2091-2. 12. Okada H et al., Properties of L-type bovine spongiform encephalopathy in intraspecies passages. Vet Pathol 49(5), 819-823 (2012) 13. Okada H et al., Immunohistochemical detection of disease-associated prion protein in the peripheral nervous system in experimental H-type bovine spongiform encephalopathy. Vet Pathol, doi:10.1177/0300985812471541 (in press) 14. K. Hasegawa, S. Mohri, T. Yokoyama. Comparison of the local structural stabilities of mammalian prion protein (PrP) by fragment molecular orbital calculations. Prion (in press). b) International Conference 1. K. Masujin, R. Miwa, H. Okada, S. Mohri, T. Yokoyama. Comparative analysis of Japanese and other countries L-type BSE prions. Prion 2012, Amstrdam, May 10-12, 2012. 2. J. Jacobs, J. Langeveld, T. Yokoyama, O. Andreoletti, J. Hope, A. Bossers, L. van Keulen, T. Baron. Ovine field CH1641 like scrapie cases do differ from classical scrapie and BSE–Western blotting studies on PrPres. Prion 2012, Amstrdam, May 10-12, 2012. 3. T. Yokoyama, G. Dorj, H. Okada, K. Miyazawa, K. Masujin, S. Mohri. Western blotting detection of abnormal prion protein (PrPSc) from formalin-fixed paraffin-embedded (FFPE) tissues–Immunoreactivity of mab discriminate sheep scrapie types. Prion 2012, Amstrdam, May 10-12, 2012 4. M. Kuroda, J. Kono, Y. Sato, T. Ohashi, T. Yokoyama, Y. Ushiki, T. Onodera, M. Yukawa. Application of micro-ELISA for detection of prion protein from small volume of sample. Asian Pacific Prion Symposium 2012 (APPS 2012). Yokohama, July 29-30, 2012 5. K. Kasai, A. Hirata, T. Ohyama, K. Nokihara, T. Yokoyama, S. Mohri. Novel assay with fluorescence-labelled PrP peptides for differentiating L-type atypical and classical BSEs, and scrapie. Asian Pacific Prion Symposium 2012 (APPS 2012). Yokohama, July 29-30, 2012 6. G. Dorj, H. Okada, K. Miyazawa, K. Masujin, S. Mohri, T. Yokoyama. Retrospective analysis of sheep scrapie by western blotting with formalin-fixed paraffin-embedded (FFPE) tissues. Asian Pacific Prion Symposium 2012 (APPS 2012). Yokohama, July 29-30, 2012. 7. Y. Nagasawa, Y. Takahashi, T. Hondo, H. Watanabe, S. Terada, C. Saito, S. Someya, K. Watanabe, S. Ohwada, H. Kitazawa, M. Imamura, T. Yokoyama, S. Sakaguchi, N. Nishida, S. 4 Annual reports of OIE Reference Centres, 2012 OIE RL for « Bovine Spongiform Encephalopathy » – « Takashi Yokoyama » – « Japan » Mohri , H. Aso. Prion protein binding proteins of bovine intestine M cell. Asian Pacific Prion Symposium 2012 (APPS 2012). Yokohama, July 29-30, 2012. 8. S. Fukuda, T. Fujii, S. Kageyama, H. Okada, T. Yokoyama, Y. Murayama. Preclinical detection of PrPSc in eyes of BSE-affected cattle. Asian Pacific Prion Symposium 2012 (APPS 2012). Yokohama, July 29-30, 2012. 9. T. Yokoyama Atypical bovine spongiform encephalopathies. The 16th Annual meeting of Japanese society for neurovirology. Tokyo, August 30-31, 2012. 1) 10. M. Imamura, N. Kato, M. Yoshioka, H. Okada, Y. Iwamaru, Y. Shimizu, S. Mohri, T. Yokoyama, Y. Murayama. Proteinase K- and heat-treated insect cell lysate effectively stimulates in vitro high-fidelity replication of baculovirus-derived recombinant scrapie and BSE prions. Asian Pacific Prion Symposium 2012 (APPS 2012). Yokohama, July 29-30, 2012. 11. Y. Iwamaru, T. Takenouchi, M. Imamura, S. Mohri, T. Yokoyama, H. Kitani. Prion-mediated cytotoxicity in a differentiated neurosphere culture. Asian Pacific Prion Symposium 2012 (APPS 2012). Yokohama, July 29-30, 2012. 15. Did your laboratory provide scientific and technical training to laboratory personnel from other OIE Member Countries? Yes No a) Technical visits: ................................................................ 3 b) Seminars: ......................................................................... 0 c) Hands-on training courses: .............................................. 0 d) Internships (>1 month): ................................................... 1 Type of technical training provided (a, b, c or d) Country of origin of the expert(s) provided with training No. participants from the corresponding country a China 3 d Indonesia 1 16. Does your laboratory have a Quality Management System certified according to an International Standard? Yes 17. No Is your laboratory accredited by an international accreditation body? Yes No 18. Does your laboratory maintain a “biorisk management system” for the pathogen and the disease concerned? (See Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2012, Chapter 1.1.3 or Manual of Diagnostic Tests for Aquatic Animals 2012, Chapter 1.1.1) Yes 19. No Did your laboratory organise scientific meetings on behalf of the OIE? Yes Annual reports of OIE Reference Laboratories, 2012 No 5 OIE RL for « Bovine Spongiform Encephalopathy » – « Takashi Yokoyama » – « Japan » National/ International Title of event Co-organiser Date (mm/yy) Location No. Participants International Asia pacific prion symposium 2012 APSPR (Asia pacific society for prion research) July 2012 Yokohama, Japan 100 20. Did your laboratory participate in scientific meetings on behalf of the OIE? Yes No Title of event Date (mm/yy) Location Role (speaker, presenting poster, short communications) Title of the work presented Prion 2012 05/12 Prion 2012, Amstrdam, Speaker Western blotting detection of abnormal prion protein (PrPSc) from formalin-fixed paraffinembedded (FFPE) tissues. 21. Did your laboratory exchange information with other OIE Reference Laboratories designated for the same pathogen or disease? Yes 22. No Was your laboratory involved in maintaining a network with OIE Reference Laboratories designated for the same pathogen or disease by organising or participating in proficiency tests? Yes 23. No Did your laboratory collaborate with other OIE Reference Laboratories for the same disease on scientific research projects for the diagnosis or control of the pathogen of interest? Yes No Title of the project or contact Scope Name(s) of relevant OIE Reference Laboratories Workshop on CWD CWD surveillance of wild deer OIE RL for CWD, South Korea Discussion for the establishment of mutual collaboration CWD surveillance of wild deer OIE RL for CWD, Canada 24. Did your laboratory organise or participate in inter-laboratory proficiency tests with laboratories other than OIE Reference Laboratories for the same disease? Yes 25. Did your laboratory place expert consultants at the disposal of the OIE? Yes 6 No No Annual reports of OIE Reference Centres, 2012