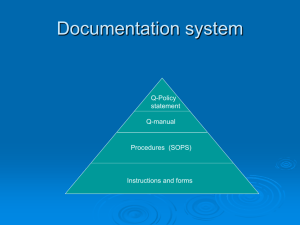
Quality Management System (QMS)
advertisement

• It was found in 1946 in Geneva, Switzerland. • its main purpose is to promote the development of international standards to facilitate the exchange of goods and services worldwide. •ISO composed of more than 90 member countries. •United states representative is the American National Standard Institute (ANSI) •ISO Technical Committee (TC) 176 developed a series of international standards for quality systems which were first published in 1987. •It is intended to be advisory and were developed for use in two-party contractual situations and internal auditing. In a two – party system, the supplier of a product or service would develop a quality system that conformed to the standards. The customers would then audit the system for acceptability. This two-party system results in both the supplier and customer having to participate in multiple audits, which can be extremely costly. This practice is replaced by a third-party registration system, who is a registrar. This involves the assessment and periodic surveillance audit of the adequacy of the supplier’s quality system and confirms to the registrar’s interpretation of the standard, then issues a certificate of registration to the suppliers. This registration ensures customers or potential customers that a supplier has a QMS in his product and service •Primary reason it that the customers or marketing are suggesting or demanding compliance to a quality system. •Needed improvement in processes or system • a desire for global deployment of products and services. The purpose of these standards is to assist organizations of all types to implement and operate effective quality management system to achieve customer satisfaction continuously. These standards provide a vehicle for consolidating and communicating concepts in the field of quality management that have been approved by an international committee of representatives from national standards bodies. The primary users of the standards are intended to be organizations acting as either customers or suppliers. Three standards of the series are ISO 9000:2000 - Quality Management Systems(QMS) – fundamentals and vocabulary discusses the fundamental concepts related to the QMS and provides the terminology used in the other two standards. ISO 9001:2000 – Quality Management Systems (QMS) – requirements is the standard used for registration by demonstrating conformity of the QMS to customers regulatory and the organization’s own requirements. ISO 9004 : 2000 – Quality Management Systems (QMS) – guidelines for performance improvement provides guidelines that an organization can use to establish a QMS focused on improving performances. The standard has 8 clauses: Scope , Normative References, Definitions, Quality Management Responsibility, Resource Management, product and/or service Realization and Measurement, Analysis and Improvement. This is called as Model of a Process-Based quality Management system. The application of a system of process without an organization, together with their identification and interactions and the managing of these processes, if referred to as the process approach. 1. Scope : The purpose of the ISO 2. Normative Reference: the fundamentals and vocabulary are a normative reference that provides applicable concepts and definitions. 3. Terms and Definitions: supply chain is defined as Supply Organization Customer 4. Quality Management System (QMS) 4.1 General Requirements •Identify the need •Determine their sequence and interaction. •Determine criteria and methods for effective operation and control of these processes. •Ensure the availability of resources and information •Monitor, measure and analyze these processes •Implementation actions to achieve planned results and continual improvement of these processes. 4.2 Documentation: 4.2.1 General: includes •Statements of a quality policy and quality objectives. • a quality manual •Required documented procedures •Needed documents to ensure effective planning, operation, and control processes •Required records. •A proper work instruction or procedure should be given. 4.2.2 Quality Manual : includes •The scope of the QMS •The documented procedures or reference to them •A description of the interaction among the QMS processes.d 4.2.3 Control of Documents •approve documents prior to use •Review, update and re-approve as necessary •Identify the current revision status. •Ensure that current versions are available at the point of use •Ensure that documents are legible and readily identified •Identify and distribute documents of external origin •Provide for the prompt removal of obsolete documents and suitably identify any that may be retained 4.2.4 Control of records: it should be legible, readily identifiable, and retrievable. It should be established and maintained to provide evidence of conformity to requirements and effective operation of the QMS. 5. MANAGEMENT RESPONSIBILITY: 5.1 Management Commitment : to the development, implementation and continual improvement of the QMS by •Communicating the need to meet customer, legal, and regulatory expectations •Establishing a quality policy •Ensuring the quality objectives are established •Conducting management reviews •Ensuring the availability of resources. 5.2 CUSTOMER FOCUS Top management shall ensure that customer requirements are determined and met with the aim of enhancing customer satisfaction. 5.3 QUALITY POLICY Top Mgmt shall insure that the quality policy •Is appropriate to the organization’s purpose or mission •Includes a commitment to comply with requirements and continually improve the effectiveness of the QMS •Provide a framework for establishing and reviewing the quality objectives •Is communicated and understood within the organization •Is reviewed for continuing stability 5.4 PLANNING 5.4.1 Quality Objectives 5.4.2 Quality Management System Planning 5.5 RESPONSIBILITY, AUTHORITY AND COMMUNICATION 5.5.1 Responsibility and Authority 5.5.2 Management Representative: it includes •ensuring that processes needed for the QMS system are established, implemented and maintained. •Reporting to top management on the performances of the QMS and any need for improvement •Ensuring the promotion of awareness of customer requirement throughout the organisation 5.6 MANAGEMENT REVIEW 5.6.1 GENERAL : top mgmt shall review the QMS at planned intervals to ensure its continuing suitability, adequacy and effectiveness. Improve its opportunities and record it. 5.6.2 REVIEW INPUT : includes information •Results of audits •Customer feedback •Process performance and product conformity •Status of corrective and preventative performance •Follow-up actions from previous management reviews •Changes that could affect the QMS •Recommendations for improvement 5.6.3 REVIEW OUTPUT : it is related to •Improvement of the effectiveness of the QMS and its processes •Improvement of the product related to customer requirements •Resource needs. These are used by the top mgmt as the input for the improvement opportunities. 6.RESOURCE MANAGEMENT 6.1 Provision of Resources : the organisation shall determine and provide the resources needed •To implement and maintain the QMS and continually improve its effectiveness •To enhance customer satisfaction by meeting customer requirement. 6.2 HUMAN RESOURCES 6.2.1 GENERAL : proper and appropriate education, training, skills and experience. 6.2.2 COMPETENCE,AWARENESS AND TRAINING:the org. shall •determine the necessary competence for personnel performing work affecting product quality •Provide training or take other actins to satisfy these needs •Evaluate the effectiveness of the actions taken •Ensure that its personnel are aware of the relevance and importance of their activities and how they contribute to the achievement of the quality objectives •Maintain appropriate records of education, training, skills and experience 6.3 INFRASTRUCTURE : it includes •Buildings, workspace and associated utilities •Process equipment (both hardware and software) •Supporting services(such as transport or communication) 6.4 WORK ENVIRONMENT The org. shall determine and manage the work environment needed to achieve conformity to product requirements. Good work environment will influence on employee motivation, satisfaction and performance. 7. PRODUCT REALIZATION 7.1 PLANNING OF PRODUCT REALIZATION •Quality objectives and requirements for the product •The need to establish processes, documents, and provide resources specific to the product •Required verification, validation, monitoring, inspection and test activities specific to the product and the criteria for product acceptance •Records needed to provide evidence that the realization processes evidence that the realization processes and resulting product or service meet requirements. 7.2 CUSTOMER-RELATED PROCESSES 7.2.1 Determination of Requirements Related to the Product. •Requirements specifies by the customer, including the requirements for delivery and post-delivery activities. •Requirements not stated by the customer but necessary for specified or intended use where known •Statutory and regulatory requirements related to the product •Any additional requirements determined by the organization. 7.2.2 Review of Requirements Related to the product. •Product requirements are defined •Contract or order requirements differing from those previously expressed are resolved •The organization has the ability to meet the defined requirements. 7.2.3 Customer Communication: relating to •Product information •Inquiries, contracts or order handling including amendaments •Customer feedback including customer complaints. 7.3 DESIGN AND DEVELOPMENT 7.3.1 Design and Development Planning: the org. shall determine •The design and development stages •The review, verification and validation that are appropriate to each design and development stage •The responsibilities and authorities for design and development. 7.3.2 Design and Development Inputs: •Functional and performance requirements •Applicable statutory and regulatory requirements •Where applicable, information derived from previous similar designs •Other requirements essential for design and development 7.3.3 Design and Development Output: it includes •Meet the input requirements for design and development •Provide appropriate information for purchasing, production and for service provision •Contain or reference product acceptance criteria •Specify the characteristics of the product that are essential for its safe and proper use. 7.3.4 Design and Development Review: •To evaluate the ability of the results of design and development to meet requirements •To identify any problems and propose necessary actions. 7.3.5 Design and Development Verification: •planned input met the output •Records are verified and necessary actions shall be maintained. 7.3.6 Design and Development Validation: Validation and verification confirms through objective evidences that the requirements for a specific intended use have been fulfilled. 7.3.7 Control of Design and Development Changes: the changes are identified and records are maintained. The changes shall be reviewed, verified and validated and approved before implementation. 7.4 PURCHASING 7.4.1 Purchasing Process. Criteria for selection, evaluation and reevaluation shall be established. Records of the results are maintained. 7.4.2 Purchasing information. •Requirements for approval of product, procedures, processes and equipment •Requirements for qualification of personnel •Qms requirements. 7.4.3 Verification of Purchased Product : Org. shall establish it. 7.5 PRODUCTION AND SERVICE PROVISION 7.5.1 Control of Production and Service Provision: it include •The availability of information that describes the characteristics of the product. •The availability of work instructions as necessary •The use of suitable equipment •The availability and use of monitoring and measuring devices •The implementation of monitoring and measurement •The implementation of release, delivery and post-delivery activities. 7.5.2 Validation of Processes for Production and Service provision : it shall demonstrate the ability of these processes to achieve planned results. 7.5.3 Identification and Traceability : the org. shall identify the product status with respect to monitoring and measurement requirements where traceability is a requirement the org. shall control and record the unique identification of the product. 7.5.4 Customer Property : the org. identify, verify protect and safeguard customer property provided for use or incorporation into the product. If any is lost, damaged or otherwise found to be suitable for use this shall be reported to the customer and records maintained. 7.5.5 Preservation of Product: it includes identification, handling, packaging, storage and protection. The org. shall preserve the conformity of product during internal processing and delivery to the intended destination. 7.6 CONTROL OF MONITORING AND MEASURING DEVICES 8 MEASUREMENT, ANALYSIS AND IMPROVEMENT 8.1 General it need •To demonstrate conformity of the product •To ensure conformity of the QMS •To continually improve the effectiveness of the QMS 8.2 Monitoring and Measurement : 8.2.1 Customer Satisfaction 8.2.2 Internal Audit 8.2.3 Monitoring and Measurement of Processes 8.2.4 Monitoring and Measurement of product and services 8.3 Control of Nonconforming product : the org. shall ensure that product which does not conform to product requirements is identified and controlled to prevent its unintended use or delivery. 8.4 Analysis of Data : the org. shall determine, collect and analyze appropriate data to demonstrate the suitability and effectiveness of the QMS and to evaluate where continual improvement of the effectiveness of the QMS can be made. 8.5 Improvement: 8.5.1 Continual Improvement: the org. shall continually improve the effectiveness of the QMS through the use of the quality policy, quality objectives audit results, analysis of data, corrective and preventive actions, and managements review. 8.5.2 Corrective Actions: 8.5.3 Preventive Actions: