by the Encapsulated Cells…
advertisement

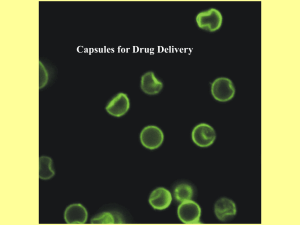
Safe Harbor Statement This presentation may include statements that constitute “forward looking statements,” which are often characterized by the terms “may,” “believes,” “protects,” expects” or “anticipates” and do not reflect historical facts. Forward-looking statements involve risks, uncertainties and other factors that may cause actual results, performance or achievements of Nuvilex, Inc. and its subsidiaries to be materially different from those expressed or implied by such forward-looking statements. Forward-looking statements speak only as of the date the statement was made. Nuvilex does not undertake, and specifically declines, any obligation to update any forward-looking statements. Factors that may affect forward-looking statements and Nuvilex’s business generally include, but are not limited to: (i) the risk factors, cautionary and other statements set forth in Nuvilex’s periodic filings with the Securities and Exchange Commission available at www.sec.gov; and (ii) other factors that Nuvilex is currently unable to identify or quantify, but may exist in the future. Corporate Profile Exchange: OTC.QB Stock Symbol: : NVLX Recent Close: $0.45 Fiscal Year End: April 30 Shares Outstanding: 638M Market Cap: $250M 52-Wk Range: $0.62 - $0.04 a/o (03/04/14) Introduction In 2013, Nuvilex acquired the exclusive, worldwide rights to use a cellulose-based live-cell encapsulation technology, known as Cell-ina-Box™, for the development of treatments for all types of cancer and for diabetes. Nuvilex Pipeline Pipeline Live-Cell Encapsulation plus Ifosfamide (IFEX®) for Pancreatic Cancer Pre-Clinical Phase 1 Phase 2 Phase 3 Live-Cell Encapsulation plus Cyclophosphamide (CYTOXAN®) for Breast Cancer Pre-Clinical Phase 1 Phase 2 Phase 3 Live-Cell Encapsulation plus Insulin-Producing Cells for Diabetes Pre-Clinical Phase 1 Phase 2 Phase 3 Live-Cell Encapsulation plus Cannabinoids for Brain and Pancreatic Cancers Pre-Clinical Phase 1 Phase 2 Phase 3 Cell-in-a-Box™ Live-Cell Encapsulation The Cell-in-a-Box Encapsulation Process Cells are mixed with a proprietary polymer (Gel8, polymer A) and the mixture is physically morphed into beads. The beads are allowed to fall into a polymerization bath containing polymer B. Almost immediately, a complex forms that produces the capsule membrane which grows from the outside toward the center, trapping groups of cells inside. Each capsule contains approximately 10,000 cells, but the capsules can be made larger or smaller and can contain more or fewer cells, depending on the type and size of the cells that are encapsulated. Cells do not change properties during encapsulation. Cell-in-a-Box Capsules… Microscopy 0.7 mm View of a Single Capsule Capsule Cross Section Showing Cells Cell-in-a-Box Encapsulation… Properties The capsules are about the size of the head of a pin. The capsules serve as protective “cocoons” for the cells inside them. Almost any kind of live cells can be encapsulated using the Cell-in-a-Box technology; only minor “tweaking” of the process is required to change from one cell type to another. The choice of cells encapsulated depends entirely upon their intended use. The number of cells per capsule depends upon the type of cells encapsulated. Since the capsules are made mainly of cellulose, they are bio-inert; no appreciable degradation of the capsules is seen after >2 years in the human body. Cell-in-a-Box Encapsulation… Properties The capsules do not cause any inflammatory response in, or damage to, tissues near where they are placed in the body. Cells within the capsules remain alive and functioning for long periods of time (>2 years) within the body. Cells within the capsules are fully protected from attack (rejection) by the body’s immune system Encapsulated cells can be frozen and stored for long periods of time (>5 years) and then thawed successfully (>90% viability - an important property for long-distance shipment. The encapsulation process has been conducted according to cGMP standards. Cell-in-a-Box Encapsulation… Advantages Other types of live-cell encapsulation use biological materials such as alginate (from seaweed) and chitosan; such materials must be extensively purified to remove contaminating substances, e.g. endotoxins, before they are used - this is not necessary with Cell-in-a-Box. As capsules made of biological material degrade, they can also elicit inflammatory responses in, and cause damage to, nearby tissues - this does not occur with Cell-in-aBox. Capsules made from such biological materials are less “robust” than Cell-in-a-Box capsules and degrade over time (a few months) in the body; Cell-in-a-Box capsules remain intact for >2 years. Long-term freezing and then thawing of capsules made from biological materials is problematic and, as a consequence, successful long distance shipping can be affected - this is not a problem with Cell-in-aBox. As a consequence of capsule degradation, the cells inside the capsules can escape and be attacked by the hosts’ immune systems - this does not occur with Cell-in-a-Box. Unlike Cell-in-a-Box encapsulation, some other forms of live-cell encapsulation have not been carried out according to cGMP standards. Cell-in-a-Box … Cancer Use First… Capsules Containing Cancer Prodrug–Activating Cells are Implanted in or near the Tumor… Immune System Cells Immune System Cells Nutrients Waste Products A Single Capsule Containing Cancer Prodrug-Activating Cells Cell-in-a-Box … Cancer Use Then – 1) Cancer Prodrug is Administered… Inactive Prodrug Molecules Capsules Containing Prodrug-Activating Cells Activated Drug Molecules 2) Cancer Prodrug is “Activated” by the Encapsulated Cells… 3) Tumor Cells are Destroyed! Pancreatic Cancer… Statistics and Characteristics Pancreatic cancer is the fourth leading cause of cancer-related deaths in the Western world. In 2013, ~45,000 new cases and ~38,000 deaths were expected in the US alone. Pancreatic cancer is difficult-to-treat because it is usually not diagnosed until it is at an advanced stage of development. The only single-drug treatment for advanced pancreatic cancer is Gemzar® (gemcitabine), approved by the FDA in 1996. Since 2000, more than 30 Phase 3 clinical trials have been conducted to try to improve upon Gemzar®. Less than 5 have been successful. Pancreatic Cancer… Combination Chemotherapies In 2005, gemcitabine + Tarceva® was approved as first-line therapy. This combination was approved, in part, because, compared to gemcitabine plus placebo, it increased the median survival from 6.0 to 6.4 months and the 1-year survival rate from 19% to 24%. In September, 2013, the combination of gemcitabine plus Abraxane® was approved by the FDA and replaced gemcitabine + Tarceva® as the “gold standard” for the disease. As compared to gemcitabine alone, the gemcitabine + Abraxane® combination increased the median survival from 6.7 to 8.5 months and the 1-year survival rate by about 59%. The use of both combinations is associated with serious side effects. Phase ½ Pancreatic Cancer Trial… Procedures Live cells containing the drug-activating enzyme CYP2B1 were encapsulated using the Cell-in-a-Box technology. Using radiography, a single implantation of 300 capsules (about 10,000 cells/capsule) was made in the blood supply near the pancreas, and therefore the tumor. Patients received two courses of chemotherapy with the anti-cancer prodrug* ifosfamide (Ifex®), administered intravenously at 1/3 of the “normal” dose usually employed in treating other types of cancer. -------------* An anti-cancer prodrug requires “activation,” usually by some enzyme, before it can “kill” cancer cells. Phase ½ Pancreatic Cancer Trial… Procedures Fourteen evaluable patients with advanced, inoperable pancreatic cancer were treated. Quality-of-life data were collected from all 14 patients. Radiography Placement of the Catheter Capsule Implantation Post-Capsule Implantation Vessel recovery Phase ½ Pancreatic Cancer Trial… Tumor Response CT Scans of Pancreatic Cancer TUMOR Before Treatment 20 Weeks after Treatment Phase ½ Pancreatic Cancer Trial… Results Summary As compared to historical data for Gemzar®, the median survival time was increased from 23 to 44 weeks and the 1-year survival rate was increased form 18% to 36% Side effects normally associated with ifosfamide were reduced in severity – no treatment-related serious adverse events were seen – probably because only 1/3 of the “usual” dose of drug was used. No side effects were seen that could be attributed to the presence of the capsules. No “inflammation” of the tissues near the capsules was apparent. Some metastatic tumors in the liver were reduced in size. The encapsulated cells remained alive and functioning for >2 years after implantation. Phase 1/2 Pancreatic Cancer Trial… Publications Relevant literature citations: Löhr M., Hoffmeyer A., Kröger J-C., Freund M., Hain J., Holle A., Karle P., Knöfel W.T., Liebe S., Müller P., Nizze H., Renner M., Saller R.M., Wagner T., Hauenstein K., Günzburg W.H., and Salmons B. Microencapsulated cell-mediated treatment of inoperable pancreatic carcinoma. The Lancet, Vol. 357, p. 1591 (2001). Löhr M., Kröger J-C., Hoffmeyer A., Freund M., Hain J., Holle A., Knöfel W.T., Liebe S., Nizze H., Renner M., Saller R., Müller P., Wagner T., Hauenstein K., Salmons B., and Günzburg W.H. Safety, feasibility and clinical benefit of localized chemotherapy using microencapsulated cells for inoperable pancreatic cancer in a phase I/II trial. Cancer Therapy, Vol. 1, p. 121 (2003). Pancreatic Cancer Treatments - a Comparison TREATMENT Gemzar® (gemcitabine) CONTROL TRIAL PHASE MEDIAN SURVIVAL (months) ONE-YEAR SURVIVAL RATE TREATMENT CONTROL TREATMENT CONTROL 5-Fluorouracil 3 5.7* 4.2* 18%* 2%* Gemcitabine + Tarceva® 2 Gemcitabine + Placebo 3 6.4** 6.0** 24%** 19%** Gemcitabine + Abraxane® 3 Gemcitabine + Placebo 3 8.5*** 6.7*** 35%*** 22%*** Cell Encapsulation + Ifosfamide Historical Data for Gemzar® 2 11.0 5.7* 36% 18%* 1,2,3 1 Approved (see year of approval below) as first-line therapy for advanced, unresectable, or metastatic pancreatic cancer. 1 Approved by FDA in 1996. 2 Approved by FDA in 2005. 3 Approved by FDA in 2013. * From Full Prescribing Information (Package Insert) for Gemzar®. ** From Full Prescribing Information (Package Insert) for Tarceva® *** From Full Prescribing Information (Package Insert) for Abraxane® ----------------------------------------------------------NOTE: All three gemcitabine –containing treatments are associated with serious side effects. Nuvilex’s treatment has no serious effects associated with it. Preparations for Future Pancreatic Cancer Trials Contracts have been signed with Fisher BioServices and Inno Biologics. Fisher BioServices will store the drug-activating cells on a long-term basis. Inno Biologics is conducting the initial cloning of the drug-activating cells. Initial contacts have been made with CROs who will assist in all aspects of the conduct of future trials. cGMP-compliant manufacturing facilities for the Cell-in-a-Box encapsulation are being established in Thailand. Breast Cancer Cell-in-a-Box and Breast Cancer… Treatment A two-arm Phase 1/2 veterinary trial in dogs with spontaneously-occurring mammary tumors has been carried out. Arm 1 –encapsulated drug-activating cells were implanted in the tumors and then the dogs were treated with cyclophosphamide. Arm 2 – the dogs were treated with cyclophosphamide alone. The anti-cancer prodrug cyclophosphamide was used instead of ifosfamide because cyclophosphamide is currently used in breast cancer treatment regimens. The cells that were encapsulated were the same as those used in pancreatic cancer trials because ifosfamide and cyclophosphamide are converted to their cancer-killing forms in the same way. Cell-in-a-Box and Breast Cancer… Results The capsules were well tolerated; there were no serious “safety” issues. Significantly greater tumor shrinkage seen in the dogs in “Arm 1” than in those in “Arm 2.” One dog had two tumors; one tumor was treated with encapsulated cells + cyclophosphamide whereas the other tumor was treated with cyclophosphamide alone. The tumor treated with encapsulated cells + cyclophosphamide shrank by 70% as compared to the14% shrinkage seen in the tumor treated with drug alone. Other Cancers and Treatments Cell-in-a-Box: What Else for Cancer? Other cancers: Breast Liver Head and neck Prostate Other “solid” tumors that are accessible Cells that activate more than one anticancer drug could be encapsulated; by doing so, combination chemotherapies may be accomplished. Cells that produce anti-angiogenic factors could be encapsulated. These may be useful in combination with surgical resection/debulking. Diabetes Diabetes… Statistics, Market Size, and Characteristics About 370 million people have been diagnosed with the disease worldwide and approximately185 million remain undiagnosed. The market for diabetes drugs and devices has been projected to exceed $114 Billion by 2018. The serious, debilitating and even deadly complications from diabetes have become a serious “drain” on medical resources. Complications from diabetes include heart disease, vision loss, kidney disease and nerve damage. Diabetes is characterized by sustained high levels of glucose (a source of energy for cells) in the blood. Blood glucose levels are regulated by insulin produced by the “islet cells” of the pancreas. Diabetes… The Problem and a Possible solution Type 1 (juvenile-onset) diabetes – the pancreas cannot produce enough insulin and daily insulin administration is required. Type 2 (adult-onset) diabetes - the pancreas may produce insulin, but the insulin produced is not able to “do its job” (insulin resistance). Type 2 diabetes can be controlled by modifications in diet, exercise and diabetes medications. Over time, type 2 diabetics may need daily insulin administration. To avoid the necessity for daily injections of insulin, pancreatic islet cell (insulinproducing) transplantations have been studied. Transplantation of Encapsulated Xenographic Islet Cells To preclude the necessity for lifelong administration of immunosuppressive drugs if “naked” xenographic islet cells are transplanted, these cells have been encapsulated using alginate. However, the limited lifespan (in the body) of alginate-based capsules and the deleterious consequences that can result from their breakdown may be problematic in terms of developing a long-lived treatment for diabetes. Encapsulation of insulin-producing cells, rather than xenographic pancreatic islet cells, using the Cell-in-a-Box technology may circumvent most, if not all, of the above problems. Studies with Cell-in-a-Box and Insulin-producing Cells Conclusions from these studies include: Through the use of the Cell-in-a-Box encapsulation technology, a type of “artificial pancreas” was created. The fact that the implanted insulin-producing cells were not attacked by rats’ immune systems testified to long-term protective capability of Cell-in-a-Box’s cellulose-based capsules. Two of the major problems associated with pancreatic islet cell transplantations are avoided by using Cell-in-a-Box because: (a) an adequate supply of insulin-producing cells is available; (b) no potent immunosuppressive drugs are required. The Company Medical Marijuana Sciences, Inc. was established in early 2013 as a wholly-owned subsidiary of Nuvilex, Inc. The initial mission of Medical Marijuana Sciences, Inc., is to develop treatments for two of the deadliest forms of cancer, namely pancreatic cancer and brain cancer, by using constituents of Cannabis (cannabinoids). The cellulose-based live-cell encapsulation technology licensed (worldwide, exclusive) by Nuvilex and known as Cell-in-a-Box™ will play a major role in the development of such treatments. Background Since the early 1970s, cannabinoids have been known to alleviate the pain and/or nausea and vomiting associated with serious diseases such as cancer. Several drugs have been approved by the FDA and drug regulatory authorities in other countries for pain and/or nausea and vomiting. The best known cannabinoids are tetrahydrocannabinol, or THC, and cannabidiol, or CBD. CBD does not have psychotropic effects associated with it. Accordingly, plants rich in CBD and low in THC have been proposed as being most useful for medicinal purposes. In 2003, the US Government, through its Department of Health and Human Services was granted a major patent (US Patent #6330507) titled “Cannabinoids as Antioxidants and Neuroprotectants” that deals with the use of cannabinoids in treating neurological damage from strokes, trauma, or from neurological diseases (Alzheimer’s, Parkinson’s, etc.) Initial Targets for Cannabinoids + Cell-in-a-Box Initially, treatments for two of the deadliest and most difficult-to-treat cancers, those of the pancreas and brain, will be targeted for development. Pancreatic Cancer - To date, only three chemotherapy treatments have been approved by the FDA for the treatment of advanced, inoperable pancreatic cancer: – Gemzar® (gemcitabine; Eli Lilly) in 1996 – gemcitabine + Tarceva (Genentech) in 2005 – gemcitabine + Abraxane in 2013 The effectiveness of all three treatments is limited by side effects. Cannabinoids such as CBD and THC have been shown to be effective against pancreatic cancer in preclinical studies. A Pancreatic Cancer Treatment 1. Cell-in-a-Box™ capsules containing cells that have an enzyme that activates the inactive prodrugs THCA and/or CBDA are implanted in or near the tumor 3. 4. 2. The inactive prodrugs THCA and/or CBDA may be delivered by a series of IV infusions or PO via raw plant extracts Cancer-killing THC and/or CBD are produced Tumor cells are killed Initial Targets for Cannabinoids + Cell-in-a-Box Brain Cancer In the U.S. in 2013, more than 23,000 new cases of cancers of the brain and nervous system will be diagnosed and more than 14,000 people will die from them. Most anticancer drugs are not effective against brain cancer because they do not cross the “blood-brain barrier” and so cannot attack the tumor. Cannabinoids may be good candidates against brain cancer, particularly glioblastoma multiforme, because they readily cross the “blood-brain” barrier. According to the American Cancer Society, only 11 drugs are even marginally effective against brain cancer. Development of a Possible Cancer Treatment The process for developing a treatment would include: First – Develop a cell line with high activity of a decarboxylase enzyme capable of converting CBDA and THCA into CBD and THC, respectively. Second – Encapsulate these cells using the Cell-in-a-Box™ technology. Third – Implant the Cell-in-a-Box™ capsules in, or near, the tumor. Fourth – Administer the prodrugs CBDA and/or THCA orally or intravenously, possibly at low dosage to minimize deleterious side effects (e.g.,the psychoactive effects associated with THC). Advantages of Cannabinoids + Cell-in-a-Box For Patients: The treatment uses simple radiographic implantation of Cell-in-a-Box™ capsules. The capsules are bio-inert and the encapsulated cells can remain alive and functioning for long periods of time in the body. THCA and CBDA are non-toxic and non-psychoactive. There is potential for home-based THCA and CBDA prodrug delivery via juiced marijuana plant extracts as an alternative to (or in addition to) clinic-based infusion. Safety and efficacy. Advantages of Cannabinoids + Cell-in-a-Box For Investors: Nuvilex’s treatment would employ the proprietary live-cell encapsulation technology with the encapsulated cells having the capability to activate cannabinoids via a decarboxylase enzyme. The product would contain no Schedule I compounds thus avoiding legal issues. The potential exists for home-based cannabinoid prodrug delivery via juiced marijuana plant extracts as an alternative to (or in addition to) clinic-based IV infusion. Cannabinoid-based treatments may prove useful against a variety of solid tumors. Intellectual Property Patents associated with our acquisition of the worldwide, exclusive rights to use the Cell-ina-Box live-cell encapsulation technology for the development of disease treatments are: LP-AN-01-PCT / WO9701357 “Encapsulated Cells Producing Retroviral Particles” - Granted in 37 countries - 31 claims - Describes the production of the capsules and their use in treating cancer - US Patent # 6,776,985 LP-AN-02-PCT / WO9735994 “Encapsulated Cells Producing Cytochrome P450” - Granted in 37 countries - 17 claims - Describes the encapsulation of cells with elevated levels of the prodrug-activating cytochrome P450 enzyme and the use of the encapsulated cells in treating cancer in combination with prodrugs activated by this enzyme - US Patents # 6,893,634 and # 6,540,995 The Nuvilex Management Team Kenneth L. Waggoner, - Chief Executive Officer and President Patricia Gruden, - Chairman of the Board and Chief Financial Officer Dr. Robert F. Ryan - Chief Scientific Officer Dr. Gerald W. Crabtree - Chief Operating Officer Questions? Investor Relations Dillon Heins CorProminence 377 Oak Street Concourse 2 Garden City, NY 11530 Office: 218-839-9051 Email: dillonh@corprominence.com