Final Exam Review
advertisement

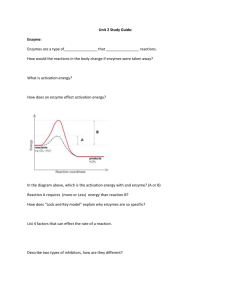
Name: ______________________________________ Date: _____________________ Period: _____________ Biology A – Trimester Exam Review Nature of Science 1. What are the steps of the scientific method? 2. Experiments typically have two variables – an independent variable and a dependent variable. What is the difference between these two? Characteristics of Life 3. Name and define the eight characteristics of life that we discussed in class. Classification 4. What is the purpose of classifying organisms? 5. What are the 8 levels of classification, beginning with the most general and going to the most specific? 6. Explain what is a dichotomous key and be able to use it. Introduction to Biochemistry 7. Define each of the following terms: Atom Element Nucleus (of an atom) Proton Neutron Electron 8. What are the four most abundant elements found in living things? Bonding in Biology 9. What are ions? 10. What is a covalent bond? 11. What is the difference between a nonpolar covalent and a polar covalent bond? 12. Explain hydrogen bonding. Water 13. Sketch three water molecules below. For each, indicate the slight charge associated with each atom and draw the hydrogen bonds between molecules. 14. What type of bond holds the atoms together in a water molecule? 15. What do the terms hydrophobic and hydrophilic mean? 16. Substances that are hydrophilic are: polar nonpolar (circle one) 17. Substances that are hydrophobic are: polar nonpolar (circle one) 18. Complete the following table about the properties of water. Property 1. 2. 3. 4. 5. What does this mean? Why is this important to life? 19. What gives water these special properties? Acids, Bases, and pH 20. Complete the following table for acids and bases. Concentration of H+ ions Concentration of OH- ions pH range Acid Base Neutral BIOCHEMISTRY 1. What is the difference between an organic and an inorganic molecule? 2. What is a dehydration synthesis reaction? 3. What is a hydrolysis reaction? 4. What is the difference between a monomer and a polymer? Carbohydrates 1. What is the general formula of a carbohydrate? ____________________ 2. Draw the general shape of a monosaccharide, a disaccharide and a polysaccharide. 3. What are mono- and disaccharides used for? 4. What is starch? Where is it typically found? What is it used for in organisms? 5. What is cellulose? Where is it typically found? What is it used for in organisms? 6. What is glycogen? Where is it typically found? What is it used for in organisms? 7. What organisms are capable of making carbohydrates? What is the process that makes these? Lipids 8. What does HYDROPHOBIC mean? 9. What elements are usually found in a lipid? 10. What are three different ways that lipids are used by organisms? 11. What are the three classes of lipids? Provide an example for each. 12. What are the characteristics of saturated fat? Give an example. 13. What are the characteristics of unsaturated fat? Give an example. Nucleic Acids 14. What is the function of nucleic acids in living organisms? 15. What is the building block (monomer) for nucleic acids? 16. What are the 3 parts of a nucleic acid? 17. Describe the shape of the DNA molecule. Proteins 18. List 3 ways that living things use proteins. 19. Give 3 examples of proteins in living organisms. 20. Proteins are chains of what smaller organic molecule? 21. How many different amino acids are there? _______________________________________ 22. What is it called when a protein unfolds? __________________________________________ 23. What conditions cause a protein to unfold? 24. How does unfolding impact the protein’s function? Draw that molecule! You will need to be able to identify pictures of each type of macromolecule and/or its building blocks. Enzymes 25. What is an enzyme? 26. What is a substrate? 27. What type of molecule are enzymes? 28. What is the active site of an enzyme? 29. Explain the lock-and-key model of enzyme activity. 30. What is activation energy? 31. How do enzymes facilitate reactions? 32. The following is an enzyme-facilitated reaction that we worked with in our labs: H2O2 What is/are the reactant(s)? What is/are the substrate(s)? What is the enzyme? catalase H2O + O2 33. What would happen if you boiled the enzyme? 34. What would happen if you froze the enzyme? 35. What would happen if you placed the enzyme in a strong acid? A strong base? Cell Theory 36. What are the three parts of the Cell Theory? Cell Structure and Function Directions: For each part of the cell listed, give the function and location within the cell. Then, check the type(s) of cells where this organelle can be found: prokaryote, animal, or plant cell. Eukaryotes Prokaryote Cell Part Function Location (Bacteria) Animal Cell Plant Cell Cell Membrane Cell Wall Chloroplast Cytoplasm Cytoskeleton Endoplasmic Reticulum Golgi Body/Apparatus Lysosome Mitochondria Nucleus Ribosome Vacuole Cellular Transport 37. What is the difference between active and passive transport? 38. Name and define the three types of passive transport. 39. What is a concentration gradient? 40. What is equilibrium? 41. Complete the following table (found in your notes!) 42. Explain the following two active transport mechanisms: Endocytosis Exocytosis Cell Cycle 1. Why do cells divide? 2. Describe the cell cycle. 3. Order the phases of the cell cycle.