lab.10 Vitamins
advertisement

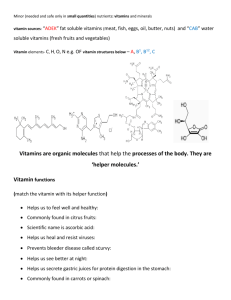
Fat Soluble Vitamins Water Soluble Vitamins Characteristics of Vitamins Vitamins are micronutrients Vitamins are essential. The roles they play in the body are very important. Most vitamins are obtained from the foods we eat. Some are made by bacteria in the intestine There is no perfect food that contains all the vitamins in the right amount. Vitamins are non-energy producing Very small amounts are needed by the body (>1 gm) Very small amounts are contained in foods. They do not contain kcalories. Vitamins are classified according to how soluble they are in fat or water. Fat Soluble Vitamins vs. Water Soluble Vitamins A, D, E, K Fat-Soluble Vitamins found in fats and oils require bile for absorption enter the lymph, then the blood held and stored in fatty tissues Needed in small amounts may reach toxic levels not readily excreted Vitamin A 3 forms in the body Retinol retinal retinoic acid collectively known as retinoids precursor: beta-carotene derived from plant foods can split and form retinol in intestine and liver Beta-carotene Dark leafy green vegetables Deep orange veggies Deep orange fruits Vitamin A function vision maintain epithelial tissue and skin support reproduction and growth Immune system Bone development Vitamin A deficiency infectious disease pneumonia, measles, diarrhea keratinization dry, rough, scaly skin night blindness Vitamin D body can make from sunlight precursor made from cholesterol production occurs in liver and kidney diseases can affect activation sources fortified food: milk, margarine, cereals, beef, eggs sun storage from the summer does not last the winter Vitamin D function part of the bone-making/maintenance team maintains blood concentrations of Ca & P Mineralization of bones raises blood calcium and phosphorus by increasing absorption from digestive tract withdrawing calcium from bones stimulating retention by kidneys deficiencies ultimately creates a calcium deficiency rickets, osteomalacia or rickets Vitamin E antioxidant defender against free radicals polyunsaturated fatty acids may reduce the risk of heart disease widespread in food easily destroyed by heat processing deficiencies rare erythrocyte hemolysis An antioxidant is a molecule that inhibits the oxidation of other molecules. Vitamin K aids in blood clotting and bone mineralization deficiency causes hemorrhagic disease sources made by bacteria in GI tract absorbed and stored in liver B complex , c Water soluble vitamins The B-complex vitamins are often associated with giving a person more energy. This is due to the fact that these vitamins each play different roles with energy metabolism in the body. When they are present in the body, they allow energy to be used more readily by the body. Since these vitamins are water soluble, they are not stored in the body like fat soluble vitamins. They dissolve in water and are excreted from the body in urine. Therefore, it is important to consume foods rich in these vitamins each day in order to fulfill the body’s need. B Complex Vitamins Co-enzymes (activate enzymes) Found in the same foods Single deficiency rare Act together in metabolism Metabolic pathways used by protein, carbohydrate, and fat B Complex Vitamins Thiamin (B1) Riboflavin (B2) Niacin (B3) Pantothenic Acid Biotin Pyridoxine (B6) Folate Vitamin B-12 B Complex Primary Functions Energy metabolism Thiamin (B-1), Riboflavin (B-2), Niacin (B-3), Pyridoxine (B-6), Biotin, Pantothenic Acid Red blood cell synthesis Folate, B12 Homocysteine metabolism Folate, B12, B6 Vitamin C Synthesized by most animals (not by humans) Decrease absorption with high intakes Excess excreted Food Sources of Vitamin C Citrus fruit Potato Green pepper Cauliflower Broccoli Strawberry Romaine lettuce Spinach Functions of Vitamin C Reducing agent (antioxidant) Iron absorption (enhances) Synthesis of collagen Immune functions Does not prevent colds, but may reduce duration of symptoms by a day Wound healing Easily lost through cooking Sensitive to heat Sensitive to iron, copper, oxygen Vitamin C Deficiency: History of Scurvy Vitamin C (ascorbic acid) deficiency leads to scurvy, a disease characterized by weakness, small hemorrhages throughout the body that cause gums and skin to bleed, and loosening of the teeth. Sailors that were out at sea for months on end would often develop scurvy unless the captain had the foresight to pack limes and other citrus fruits. In the U.S., deficiency is seen mostly in alcoholic persons with poor diets and older persons who eat poorly (no fresh fruits and vegetables) Scurvy Scorbutic Rosary Follicular Hemorrhages Vitamin C Excess Hemochromatosis Vitamin C enhances iron absorption Oxalate kidney stones Erodes tooth enamel 24 To determine ascorbic acid (vitamin C) concentration by a redox titration with potassium iodate Ascorbic acid (vitamin C) is sometimes called an .anti- oxidant. (i.e., a reducing agent!) by pharmacists and food nutritionists. iodometric titrations. In "iodometric" titrations, the analyte is first reduced with an excess of I-, producing I2 (actually, I3-) which turns blue in the presence of starch. A suitable method for the determination of vitamin C (C6H8O6) quantities is a titration with potassium iodate (KIO3). Iodine rapidly oxidizes ascorbic acid, C6H8O6, to produce dehydro-ascorbic acid, Object: To determine vitamin C (C6H8O6) by potassium iodate titration and to master Potassium iodate is used as a titrant and it is added to an ascorbic acid solution that contains strong acid and potassium iodide (KI). Potassium iodate reacts with potassium iodide, liberating molecular iodine (I2): Potassium iodide must be added in excess to keep iodine dissolved. Once all the ascorbic acid has been consumed, any excess iodine will remain in solution. Since aqueous iodine solutions are brown in colour, iodine can act as its own indicator. However, it is quite difficult to detect endpoints using iodine coloration alone, and it is more usual to add starch, which forms an intensely blue coloured complex with iodine but not with the iodide ion. The addition of acid is necessary to provide the acidic conditions required in reaction (1) above. The endpoint of the titration is the first permanent trace of a dark blue-black colour due to the starchiodine complex. Note: The end point is reached when the solution turns a permanent, dark blue colour, due to the complex formed between starch and iodine. During an iodometric titration an intermediate dark blue iodine-starch complex may form momentarily, before the iodine reacts with ascorbic acid. However, if the colour disappears upon mixing, the end point has not yet been reached. Thus, magnetic stirrers or glass rod are employed in the titration to ensure proper mixing and to facilitate the reaction of iodine with ascorbic acid. Iodometric Titrations Molecular iodine (I2) is only slightly soluble in water but adding iodide, I-, produces the "triiodide" ion (I3-) in solution. Thus, KI is almost always added when redox reactions of I2 are involved in quantitative analysis. I2(s) + I-(aq) I3(aq) Iodine iodide triiodide Starch is used as the indicator in most iodometric titrations because iodine (i.e., I3)forms an intense blue colored "starch-iodine complex." Experiment Principle 1. KIO3 is used as a titrant and it is added to an ascorbic acid solution that contains a strong acid and potassium iodide (KI). 2. KIO3 reacts with KI, liberating molecular iodine (I2): KIO3 + 5KI + 6H+ → 3I2 + 6K+ + 3H2O (1) C6H8O6 + I2 → C6H6O6 + 2I- + 2H+ (2) 31 According to the above reactions, each mole of potassium iodate added corresponds to 3 moles of ascorbic acid dehydrogenated in the sample. PROCEDURE 1. Pipette 25 ml of the provided ascorbic acid solution into a 250 ml conical flask, 2. Add 4 ml of 2M HCl, 3. Add 5 ml of potassium iodide (KI) solution and 3 ml starch solution. 4. Then titrate with the standard potassium iodate (KIO3) solution until the solution turns intense blue. Write down the standard potassium iodate (KIO3) solution volume. 5. Pipette 25 ml of an unknown ascorbic acid sample, a kind of juice, into a 250 ml conical flask, then follow the same procedure of steps 1-4 and write down the volume of the standard KIO3 solution determine the concentration (mol/ml) of ascorbic acid in the selected sample. 33 procedure