M3_3s
advertisement
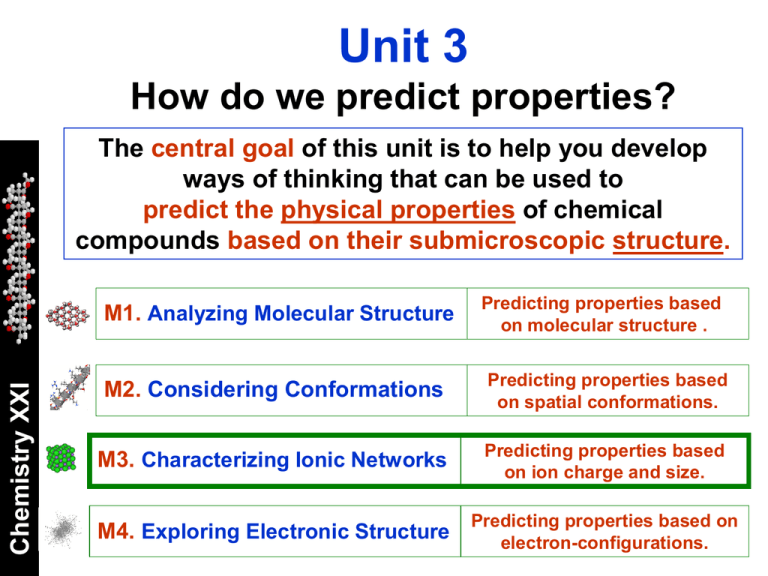
Unit 3 How do we predict properties? The central goal of this unit is to help you develop ways of thinking that can be used to predict the physical properties of chemical compounds based on their submicroscopic structure. Chemistry XXI M1. Analyzing Molecular Structure Predicting properties based on molecular structure . M2. Considering Conformations Predicting properties based on spatial conformations. M3. Characterizing Ionic Networks Predicting properties based on ion charge and size. M4. Exploring Electronic Structure Predicting properties based on electron-configurations. Unit 3 How do we predict properties? Chemistry XXI Module 3: Characterizing Ionic Networks Central goal: To explain and predict the physical properties of ionic compounds based on the charge and size of the ions present in the system. The Challenge Modeling How do I predict it? Chemistry XXI Metals can chemically react with nonmetals to form new chemical compounds. In most cases, these compounds are not comprised of molecules, but of ions arranged in solid crystalline networks. How can we make predictions about the composition of these ionic compounds? How can we predict what properties they may have? Important Differences Nonmetals Chemistry XXI Metals In general, atoms of nonmetals have higher ionizations potentials and electronegativities (c) than those of metals. Thus, nonmetals have a higher affinity for electrons than metals. From Polar to Ionic Chemistry XXI The larger the difference in electronegativity (Dc) between bonding atoms the more polar the bond. For Dc > ~1.7, the bonding electrons spend most of their time close to the nonmetal atom. Ionic Compounds Chemistry XXI The properties of the compounds that results from the reaction of metals with nonmetals are better described assuming that independent ions are formed. Metal + ion (cation) Nonmetal - ion (anion) Ionic Networks + and - ions interact with all other ions in the system, and they do not form molecules. Chemistry XXI They arrange into a crystalline network. NaCl For these compounds, the chemical formula only indicates the ratio of + to – ions in the lattice (1:1). Crystal Structures Different ionic compounds may have different crystal structures. BCC FCC Chemistry XXI CsCl NaCl Every type of crystal lattice can be built by repeating a characteristic UNIT CELL. Let’s Think Chemistry XXI Ionic compounds do not conduct electricity as solids, but they do it when melted. Solid ionic compounds shatter when struck. How would you explain these properties based on their submicroscopic structure? Charge and Size In general, many properties of ionic compounds are determined by the charge and the size of the ions in the network. r Chemistry XXI Interactions between ions are determined by Coulomb’s law. q1q2 F 2 r + Thus, it is of central importance to be able to predict the charge and size of the ions formed whenever a metal reacts with a nonmetal. Let’s Think The charge that atoms of metals and nonmetals acquire when they react with each other is determined by their electron configurations. Chemistry XXI s1 s2 p1 p2 p3 p4 p5 p6 What charges would the atoms in the highlighted families will acquire when they react with each other? Justify your reasoning. Ion Charges Chemistry XXI Based on the octet rule, we can predict that the ions formed will have full shell electron configurations. Transition Metals Ion charge (+) Chemistry XXI The prediction of ion charges for transition metals is more difficult, because electrons in the d-subshell may also be transferred to the nonmetal atoms. So, they may form a variety of ions. Atomic Number Common Less Common Naming Examples: Cu(I), Cu(II), Nickel(I) Charge Neutrality No matter what ions are formed, the ionic network that is formed has no net charge. These implies that cations (+) and anions (-) combine in ratios that ensure charge neutrality. Consider this reaction: Na(s) + Cl2(g) Chemistry XXI Na Na+ Cl ClStable Ions Sodium chloride Thus, the ionic product is NaCl 2 Na(s) + Cl2(g) What happens in this case? 2 NaCl (s) 4 Al(s) + 3 O2(g) 2 Al2O3 Al Al3+ O O2Stable Ions Thus, the ionic product is Al2O3 Aluminum oxide Let’s Think Predict the ratios in which the ions resulting from these reactions will combine: K(s) + F2(g) Mg(s) + O2(g) Chemistry XXI Na(s) + S8 (s) Ca(s) + N2(g) Write the chemical formulas for the resulting ionic compounds and balance each equation. Let’s Think During the formation of ionic compounds, atoms of metals lose electrons while those of nonmetals gain them: Chemistry XXI A – m e- Am+ B + n e- BnHow would you expect the size of each type of atom to change when becoming ions? How would you expect the periodic trend for ion sizes to be? Properties Chemistry XXI The properties of an ionic compound are determined by the electrostatic forces among its ions, and between these ions any surrounding particle (atom, ion, or molecule). Coulomb’s Law r q1q2 F 2 r + Based on Coulomb’s Law, one may expect these forces to be stronger: the larger the charge of the ions (larger q1, q2); the smaller the size of the ions (smaller r); Phase Transitions Ionic compound tend to have high melting and boiling points because electrostatic forces are pretty strong. Chemistry XXI Let’s Think Interactions Energy (KJ/mol) Ionic 400 - 4000 Covalent 150 - 1100 Metallic 75 - 1000 H-bonding 10 - 40 Dipole-Dipole 5 - 25 Dispersion 0.05 - 40 Arrange each of these sets of ionic compounds, in order of increasing melting point: NaCl, NaBr, NaF CaO, NaCl, MgO Solubility Chemistry XXI Some ionic compounds are very soluble in water, while others are almost completely insoluble. Ionic compounds that are insoluble in water tend to be comprised of ions with strong interactions among each other (large charge, small size). Electrolytes Chemistry XXI Strong electrolyte Weak electrolyte Solutions of soluble ionic compounds conduct electricity. This implies that ions can freely move across the system. Solutions of slightly soluble ionic compounds barely conduct electricity. Only a small fraction of the ions is in solution. Let’s Think Consider the following examples of soluble and insoluble ionic compounds: Chemistry XXI Soluble NaCl MgBr2 K2S CaI2 NaF AlCl3 Insoluble MgO ZnS Al2O3 FeS CrS Ni2O3 Use this data to derive a simple rule to make predictions about the solubility of ionic compounds based on ion charges. Justify your ideas using Coulomb’s Law. Polyatomic Ions There are ionic compounds in which the ions in the lattice are not necessarily atomic (one single atom), but molecular (several atoms covalently bonded). OH- Chemistry XXI Hydroxide ion SO42Sulfate ion NO3Nitrate ion PO43Phosphate ion CO32Carbonate ion NH4+ Ammonium ion Comparisons No matter the type of ion, we can still make predictions about the structure and properties of these ionic compounds based on similar ideas. NH4+ Cl- Chemistry XXI Let’s Think Ammonium Chloride NH4Cl Make predictions about the solubility of these ionic compounds: Barium Sulfate Magnesium Nitrate Calcium Phosphate Ammonium Nitrate Calcium Carbonate New Applications Chemistry XXI Materials based on ionic compounds have a wide variety of applications. Most ceramics are ionic solids that are very hard and highly resistant to corrosion and wear. Zeolites, for example, are ionic compounds of Al, Si, and O, that have a porous structure. They are widely used as filters and catalysts. Surprising Properties Ions of transition metals have unpaired electrons, and thus they exhibit magnetic properties. High-T Superconductor Ceramics Chemistry XXI Solid oxides of metals such as Fe, Mn, and Cu exhibit surprising electric and magnetic properties. Magnetite Fe3O4 3+ 2+ 1+,2+ 2- YBa2Cu3O7 Chemistry XXI Let′s apply! Assess what you know Let′s apply! Some Melting Points All common ionic compounds have melting points above 100 oC and thus are solid a room temperature Cl- K+ 776 oC NH4+ 338 oC NO3- 334 oC 170 oC CH3COO- 292 oC 112 oC Chemistry XXI Based on what you have learned: Hypothesize on the structural characteristics of ionic compounds that could be liquid at room temperature. Discuss potential applications of these types of fluids. Let′s apply! Soaps are also ionic compounds in which the anions are pretty large hydrocarbon chains. Explain: Chemistry XXI Why are soaps soluble in water? How would you expect soap ions to arrange in solution? Why do soaps help in removing grease? Chemistry XXI Come up with a question about the content of this module that you desperately want to be answered. Characterizing Ionic Networks Summary Chemistry XXI Metals can chemically react with nonmetals to form new chemical compounds. In most cases, these compounds are not comprised of molecules, but of ions arranged in solid crystalline networks. No matter what ions are formed, the ionic network that is formed has no net charge. These implies that cations (+) and anions (-) combine in ratios that ensure charge neutrality. Na+ Cl- Characterizing Ionic Networks Summary Chemistry XXI The charge that atoms acquire when they react with each other is determined by their electron configurations. The ions that are formed tend to be those with a full shell electron configuration. Many properties of ionic compounds are determined by the charge and the size of the ions in the network. These forces are stronger the larger the charge of the ions and the smaller their size. Coulomb’s Law r + q1q2 F 2 r Chemistry XXI For next class, When metals are combine they form alloys. Investigate the basic characteristics of these materials. Why is that metals conduct electricity while ionic and molecular compounds do not?




