Surgical Management Of PPH

Tips & Tricks in dealing with a surgical bleeding field
Ismail A. Al-Badawi, FRCSC
Section Head, Gynecology/Oncology & MIS
King Faisal Specialist Hospital& Research Center
Associate Professor, Alfaisal University
Pelvic Vascularity
!
Introduction
150,000 women worldwide bleed to death each year while giving birth !
10 deaths per 100,000 deliveries in developed countries !
In Developing countries = 1000: 100,000 delivery !
1/3 of women with PPH have no identified risk factors !
80 – 93 % of all deaths due to bleeding are avoidable!
Global Maternity Death – WHO 2006
Causes Of Maternal Mortality
Etiology
Hemorrhage
Embolism
P.I.H.
Infection
Cardiomyopathy
Anesthesia complic
Others
M. M.
28.7%
19.7%
17.6%
13.1%
5.6%
2.5%
12.7%
Confidential Enquiry Of Maternal Death In UK 2007
http://www.moh.gov.sa/statistics/M2005/Book%202005.pdf
Why Obstetrical bleeding ?
56 422 deliveries 16 maternal deaths overall MMR of 28.4/100 000 total births
81.25% direct obstetrical causes
18.75% indirect causes
MMR due to direct causes was 23.4/100 000 deliveries.
The trend in MMR in the unit remained almost the same over the years
Obstetric haemorrhage was responsible for 43.75% maternal deaths
Journal of Obstetrics and Gynaecology (April 2004) Vol. 24, No. 3, 259–263
Types Of Bleeding
Types Of Bleeding
Surgical :
1.
Premature Placental Separation ( Abruptio Placenta
)
.
2.
Uterine Atony .
3.
Uterine Rupture .
4.
Placenta Praevia .
5.
6.
Placenta Accreta / Percreta .
Emergency LSCS
.
Types Of Bleeding
Medical :
D. I. C ( Disseminated Intravascular Coagulopathy ) .
HELLP Syndrome !
Amniotic Fluid Embolus !
Oncology cases !
Obstetrical Hemorrhage
Maternal Deaths
1.
2.
3.
4.
5.
6.
7.
Abruptio placenta – 19 percent .
Uterine rupture – 16 percent .
Uterine Atony – 15 percent .
Coagulation disorder – 14 percent .
Placenta previa – 7 percent .
Placenta accreta – 6 percent .
Retained placenta – 4 percent .
Chichaki, et al, 1999
Causes of 763 Deaths due to hemorrhage
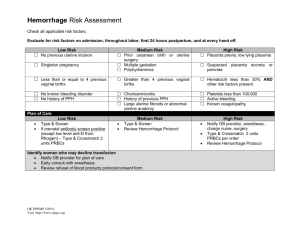
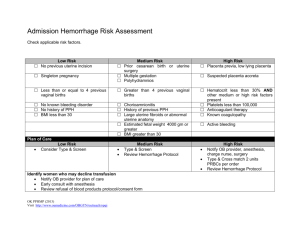
Practical Issues
Primary PPH
No Universally Accepted Definition !
Blood loss within 24 hours of delivery :
>500 mL following vaginal delivery !
>1000 mL following caesarean delivery !
Decrease in haematocrit level >10 % of prenatal value !
Any bleeding that results (or could result, if untreated) in signs of maternal haemodynamic instability !
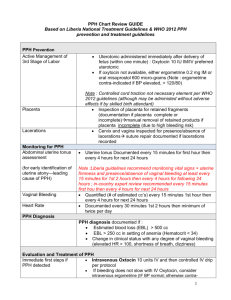
Obstetric Hemorrhage
If Hemorrhage is not controlled by medications, massage, manual uterine exploration, or suturing lacerations in the birth canal, then surgical or radiological options must be considered.
IN general, avoid a “watch and wait” approach to the bleeding patient.
- Obstetrical Hemorrhage -
Significant PPH Failed Medical Rx
Atony/Tears/ Rupture PL Previa / Accreta
B-Lynch procedure
Uterine a. ligation
Stepwize devascularization
Uterine repair
U.A.E.
Internal Iliac artery ligation
TAH
Local mattress sutures
Hemostatic balloons / Packing
U.A.E.
Stepwize devascularization
Internal Iliac artery ligation
TAH
- Obstetrical Hemorrhage -
Identify
Patients at
Risk
Multidisciplinary
“Hemorrhage protocol”
Clinical
Management of PPH
- Obstetrical Hemorrhage -
1. Identify pat. at risk
-Pl previa/accreta
-Anticoagulation Rx
-Coagulopathy
-Overdistended uterus
-Grand multiparity
-Abn labor pattern
-Chorioamnionitis
-Large myomas
-Previous history of PPH
Maternal Mortality
- Obstetrical Hemorrhage -
1. Prepare for PPH
Personnel
Drugs/Equipment
Nursing
-Anesthesia
- Surgical assistance
- Blood Bank
- ICU Team
-Methergine
-Hemabate
- Mesopristol
-Cytotec
-Colloids
- Blood products
-Surgical Instruments
-Hemostatic balloons
( Cook, S-B, Foley)
Patients at risk
Maternal Mortality
- Obstetrical Hemorrhage -
Pre-delivery management
1.
Prepare for PPH
2.- Optimize patient’s hemodynamic status
3.- Timing of Delivery
4.- Surgical planning
5.- Anesthesia /I.V. access/ invasive monitoring
6.- Modify obstetrical management
7.- Increased postpartum/post op surveillance
- Obstetrical Hemorrhage -
2. Optimize hemodynamic status
1. Acute isovolemic hemodilution !
2. Preoperative transfusion !
3. Cell Saver !
- Obstetrical Hemorrhage -
Identify
Patients at
Risk
Multidisciplinary
“Hemorrhage protocol”
Clinical management of
PPH
1.- How/Who triggers the “H.P.”
2.- Identify “The response team”
3.- Transfusion protocol
4.- Define the logistics involved
5.- Conduct drills
6.- Post-op care
Maternal Mortality
- Obstetrical Hemorrhage
-
Identify
Patients at
Risk
Multidisciplinary
“Hemorrhage protocol”
Clinical management of
PPH
1. How/Who triggers the “H.P.” ???
The OB Consultant on call for L+D !
The Head Nurse of L+D Initiate the H.P !
A single number paging system !
Over Head Calling System !
Maternal Mortality
- Obstetrical Hemorrhage -
Identify
Patients at
Risk
Multidisciplinary
“Hemorrhage protocol”
2 The “Response Team”
Clinical management of PPH
-Nursing
-Anesthesia
-Ob surgery (MFM, Gyn Onc, Ob-Gyn,)
-Intervention Radiology
-Hematology
Maternal Mortality
- Obstetrical Hemorrhage -
Identify
Patients at
Risk
Multidisciplinary
“Hemorrhage protocol”
3 - Transfusion Protocol
Clinical management of PPH
-Immediate release of O neg Blood if required
- How fast can Crossmatched blood be made available !
-Physical transport of Blood
Lab / Blood Bank !
O.R. and samples from O.R.
- Availability of different products !
Immediately
Requesting of blood products
Emergency group O Rh D negative units can be given while the patient’s blood group is being determined.
15 min after receipt of sample in blood bank
Un-crosshatched ABO group specific blood can be provided.
As soon as thepatient’s blood group has been determined a switch should be made from O
Rh (D) negative blood to ABO group specific blood. Medical staff must accept full responsibility for administration of un-crosshatched blood– the patient may have unidentified / undetected antibodies – the use of un-crosshatched blood in these patients may result in a life threatening Haemolytic Transfusion Reaction.
45 min after receipt of sample in blood bank
Fully crosshatched blood can be provided in most cases.
Tips & Tricks
Uterine Packing
The Re-emergence of uterine packing
Although uterine packing was advocated for treating PPH in the past, it fell out of use largely due to concerns of concealed hemorrhage and uterine over distension.
In recent years, however, several modifications of this procedure have allayed these concerns.
Balloon tamponade has been shown to effectively control some types of postpartum bleeding, and may be useful in several settings: placental Praevia and placenta accreta.
The re-emergence of uterine packing
The Foley catheter procedure.
A Foley catheter with a 30-mL balloon capacity is easy to acquire and may routinely be stocked on labor and delivery suites.
Using 24F Foley catheter, the tip is guided into the uterine cavity and inflated with 30 mL of saline.
Additional Foley catheters can be inserted if necessary up to 5 catheters. If bleeding stops, the patient can be observed with the catheters in place and then removed after 12 to 24 hours.
The re-emergence of uterine packing
The Sengstaken-Blakemore tube.
Originally developed for the tamponade of bleeding esophageal varices, the Sengstaken-Blakemore tube has the advantage over the
Foley catheter due to the larger capacity of its balloon !
The Sengstaken-Blakemore tube has an open tip that permits continuous drainage.
Like the Foley catheter, the Sengstaken-Blakemore tube should be guided through the cervix into the uterus and the balloon can then be inflated up to 150 cc to achieve the desired tamponade and can be removed in 12 to 24 hours.
The re-emergence of uterine packing
Bakri SOS Balloon.
It is 250 – 500 cc capacity silicon- made balloon, designed specifically to take the shape of the uterine cavity to act as a tamponade with a drainage hole in the tip of the balloon catheter to allow blood drainage if collected above the balloon.
The re-emergence of uterine packing
After C- Section:
When the balloon is placed at the time of cesarean delivery, an assistant working from below helps pull the distal end of the balloon shaft through the cervix into the vagina.
The hysterotomy incision is closed
The balloon is filled with 250 to 500 mL of sterile isotonic fluid
The distal end of the balloon is attached to a weight, such as a liter intravenous fluid bag, to ensure that a tamponade effect is maintained.
The re-emergence of uterine packing
After vaginal delivery :
A Foley catheter is inserted in the bladder to monitor urine output and reduce bladder volume !
The uterus is examined to ensure that there are no retained placental fragments !
The balloon is inserted into the uterus so that the entire balloon is past the internal cervical os !
Using a syringe, the balloon is filled with sterile saline to the desired volume—again, typically, 250 to 500 mL
Gentle downward traction is placed on the balloon stem to ensure that a tamponade effect is maintained
Selective Arterial Embolization
Selective Arterial Embolization
If the patient is stable and bleeding is not excessive, and if interventional radiology is available, then pelvic arteriography may show the site of blood loss and therapeutic arterial embolization may suffice to stop the bleeding.
Uterine Artery Embolization
Uterine Artery Embolization
Selective arterial embolization
With gelfoam pledgets, coils, or a balloon catheter, the targeted artery is occluded.
Unlike other interventions, SAE can be highly effective when coagulopathy is present.
Although long-term follow-up is unavailable for most of the reported cases, menses typically returns within 3 months, and subsequent normal pregnancies have resulted.
Selective arterial embolization
When women are at increased risk for PPH
(suspected accreta, previa), catheters can be placed prophylactically, prior to a planned C/S delivery in anticipation of need.
Many studies found that prophylactically placed catheters reduced the total blood loss and incidence of coagulopathy, compared with catheterization performed in an emergent setting.
Surgical Compression Suture
Surgical Compression Suture
In recent years, interest has surged in the surgical compression suture for treating PPH brought on by uterine atony.
B-Lynch suture initially described by Christopher
B-Lynch in 1997 has gained the most popularity .
Surgical Compression Suture
The theory behind each technique is the same:
The mechanical compression of uterine vascular sinuses prevents further engorgement with blood and continued hemorrhage.
Surgical Compression Suture
A woman meets the criteria for the
B-Lynch compression suture if bimanual compression decreases the amount of uterine bleeding by abdominal inspection.
Surgical Compression Suture
Although originally described using No. 2 chromic catgut, variations using No.1 Vicryl suture have been equally successful.
In our experience, we have also used No. 1 chromic catgut and 0 loop PDS suture with no complications noted.
There are theoretical concerns about bowel complications with PDS suture because the suture material may not completely degrade for up to 6 months.
Uterine Devascularization
Uterine
Devascularization
Uterine Devascularization
Success Rates :
Step 1: 8.7 % .
Step II : 74.8 % .
Step III : 93 % .
Internal Iliac Artery Ligation
Internal Iliac Artery Ligation
Burchell has put forward the mechanism responsible for controlling pelvic hemorrhage following ligation of internal iliac artery !
The ligation of internal iliacs greatly decreased the pulse pressure and transformed the pelvic arterial system into a venous like system with slow and sluggish blood flow.
Internal Iliac Artery Ligation
Burchell also proved that with bilateral ligation, the drop in pulse pressure was around 85 – 90 % , whereas with unilateral ligation it was 77% on the same side and 14% on the opposite side.
The rate of blood flow dropped to about 48% after ligation.
Internal Iliac Artery Ligation
Possible delayed complications like ischaemic necrosis of the gluteal muscles, weakness of gluteal muscles or bladder atony can occur up to 20 % of the cases.
Full term pregnancies with no complications like intrauterine growth retardation have been reported after bilateral internal iliac and ovarian artery ligation.
CesareanHysterectomy
Peripartum hysterectomy
often in conjunction with cesarean delivery !
In the United States, it is performed in 0.05 to 0.1 percent of all deliveries and 0.5
percent of all cesarean deliveries !
Similar results are found in Europe and Canada !
Cesarean Hysterectomy
Must be discussed with all high risk patients before delivery !
The consent must include it !
DECISION MAKING
Though , the decision to proceeds to hysterectomy should not be delayed till the patient reach critical stage or she bleed too much till she goes to DIC.
The proper timing to move to hysterectomy is vital for the well being of the patient and it must not be delayed beyond the right time in order to preserve the women fertility!
Challenges !
Anatomical changes !
Increased Vascularity !
Other Complications !
Pelvic Packing
Pelvic Packing
Very effective for wide spread oozing , raw services or for venous bleeding !
Must be done correctly to be successful !
Pack and Go back !
Packing
o
Transient compression of the aortic bifurcation against the sacral prominence can increase arterial perfusion pressure to the maternal heart, brain, and kidneys; also this will decrease loss of blood into the operative field.
Tricks are NOT only
Surgical
Three avoidable factors in most Massive Obstetric
Hemorrhage related maternal deaths
Delays in:
correction of hypovolaemia
surgical control of bleeding
diagnosis and treatment of defective coagulation
Fresh frozen plasma (FFP)
volume 200 to 250ml
contains
all coagulation factors, except FVIII(rapidly decays)
dose 15 mL/kg
Solvent detergent prepared FFP has a lower risk of transfusion transmitted infection
PHARMACOLOGICAL AGENTS THAT REDUCE
BLEEDING
Recombinant VIIa
Antifibrinolytics
Recombinant activated factor VII
off-label use
1999 first ‘off-label’ successful use: perioperative bleeding in a wounded soldier without a preexisting hemostasis disorder
2001: successful in a parturient during CS delivery
2003–2004: more case reports and small series in obstetrics
Dose: 20-120 µg/kg
No clear evidence of a dose– response relationship
Recombinant activated rFVII Registries
North European registry [Northern European FVIIa in Obstetric Haemorrhage (NEFOH)
108 cases in 9 countries -65 hospitals
5-year period (2000 -2004) single dose of rFVIIa in dose ≤7.2mg no change in bleeding or worsening
TED
MI 1 case
81%
91%
14%
4 cases
The Australian and New Zealand registry:
105 cases in 38 hospitals assessment of efficacy possible in only 94 cases median dose of 92mgkg1 single dose 78%
Positive response hysterectomy
TED encephalopathy
76% (64% to the first dose)
41% before or 21% after rFVIIa therapy
2 cases
1 (severe anoxic insults)
Registry UniSeven in the Czech Republic
80 Czech patients with life threatening PPH
2004-2009
DESIGN: Retrospective, observational, multicentre study.
RESULTS:
97.5% treatment was able to control the bleeding
66.3% only 1 dose of rFVIIa was sufficient mortality rate: 2.5%
VTE non
74.3% was administered before considering hysterectomy was able to avoid hysterectomy
Seidlová D et al Ceska Gynekol. 2010 Aug;75(4):297-305
Anti-Fibrinolytic Drugs:
Tranexamic acid
Inhibition of fibrinolysis by TXA
Plasmin
Tranexamic Acid
Lysine Binding Site
Fibrin
●
●
●
Tranexamic Acid (TXA) is a synthetic derivative of the amino acid lysine.
It has a very high affinity for the lysine binding sites of plasminogen.
It blocks these sites and prevents binding of activated plasminogen to the fibrin surface, thus exerting its antifibrinolytic effect.
TXA in elective surgery
Reduced need for transfusion
RR (95% CI)
TXA
0.61 (0.54-0.69)
0 0.4
0.8
1.2
1.6
TXA better TXA worse
2.0
TXA already recommended for use in some treatment protocols
Page 56: 1.16.13:
“Additional therapeutic options for the treatment of postpartum haemorrhage include tranexamic acid
(intravenous)…” http://www.nice.org.uk/nicemedia/pdf/IPCNICEGuidance.pdf
The most recently updated WHO treatment guidelines for PPH state that TXA may be used, but that the quality of evidence on which this recommendation is based is low, and recommend further clinical trials of
TXA in PPH.
Dr Metin Gülmezoğlu
WHO Reproductive Health Library
Post-hemorrhage care
Once bleeding has stopped
remember that the most common cause of maternal direct deaths is thromboembolism
Thromboprophylaxis:
LMWH: ASAP