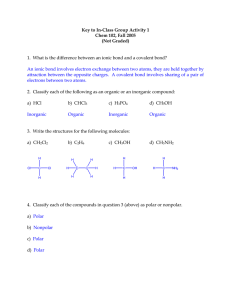
Introduction
advertisement
Introduction • Organic chemistry is the study of molecules that contain carbon • Carbon is special because: - can form 4 strong covalent bonds - can bond with other carbons to form chains and rings - can bond with a variety of other elements • Learning organic chemistry will help you understand the nature of the world around you: - pharmaceuticals, household products, plastics, etc. - essential for understanding biology and biochemistry Organic Compounds • Organic compounds are covalent • They most commonly consist of carbon bonded to H, N, O, S, F, Cl, Br, I and/or other C’s • Chemical properties are based on functional groups • Recall that the number of covalent bonds an atom will form can be predicted from its group # • Molecular shape can be predicted using VSEPR theory Example: CH4 is tetrahedral (4 electron groups) • We can extend this to larger molecules Example: C2H6 is tetrahedral around both C’s Molecular Polarity • Recall that a covalent bond can either be polar (if electronegativity difference ≥ 0.5) or nonpolar Examples: C-H is nonpolar, C-O is polar • However, molecular polarity is primarily based on shape: - Symmetrical molecules are nonpolar - Nonsymmetrical molecules are polar if: - there is at least one lone pair on central atom - or, there is at least one polar bond Examples: CCl4 is nonpolar, CHCl3 is polar Organic vs. Inorganic Compounds • In chem 101, you mostly studied inorganic compounds such as salts and inorganic acids and bases • Many inorganic compounds(NaCl, H2SO4, NH3 etc.): - are soluble in water - ionize in water (electrolytes) - have high boiling points - are not flammable • Many organic compounds (CH4, C6H6, CH3OCH3 etc.) - are insoluble in water (soluble in organic solvents) - do not ionize in water (nonelectrolytes if soluble) - have low boiling points - are flammable Functional Groups • A functional group is a part of a molecule with characteristic chemical reactivity • Organic compounds are classified by functional group - alkanes, alkenes, alkynes and haloalkanes - aromatic hydrocarbons - alcohols, thiols and ethers - aldehydes and ketones - carboxylic acids and esters - amines and amides • You will study each functional group in detail in the remaining chapters, for now just become familiar with the structures Constitutional Isomers • Isomers are compounds with the same molecular formula, but a different arrangement of atoms • There are many types of isomers • One type, constitutional isomers, have the same formula, but atoms are connected in a different order Examples: C4H10 has two constitutional isomers: CH3-CH2-CH2-CH3 and CH3-CH(CH3)-CH3 C2H6O also has two constitutional isomers: CH3-CH2-OH and CH3-O-CH3