2007-2008 Chemistry SOL Blitz
advertisement

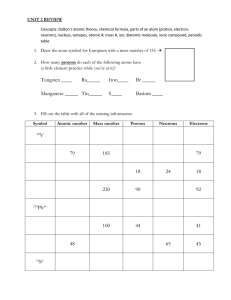
2010-2011 Chemistry SOL Blitz Question #1 • Which of the following atoms contains 30 protons, 40 neutrons and 28 electrons? • A: 70 Zn+2 • B: 70 Zn-2 • C: 40 Zn+2 • D: 30 Zr-2 Answer to Q#1 • Letter A: 70 Zn+2 is the answer. • Why? The format for chemical symbols is A Xcharge z • Remember, A = mass, which equals protons + neutrons • Z = atomic number = protons • Charge = protons – electrons Question #2 • The element boron has only two stable isotopes. One stable isotope has a mass number of 10 and the other has a mass number of 11. Which of the following could be the atomic weight of the element? • • • • A: B: C: D: 9.5 10.8 11.7 12.4 Answer to Q#2 • Letter B: 10.8 is the answer • Why? The mass of an element on the periodic table is the weighted average of all the isotopes of that element Question #3 • • • • • An atom of Argon—40 contains— A: 18 protons and 18 neutrons B: 18 protons and 22 neutrons C: 18 protons and 40 neutrons D: 20 protons and 20 neutrons Answer to Q#3 • Letter B: 18 protons and 22 neutrons • Why? The 40 in Argon-40 is the mass of the isotope • Remember mass = protons + neutrons (electrons have no mass) • Argon has an atomic number of 18, so there 18 protons Isotope/ Ion Quick Review • Isotopes of an element have the same atomic number (protons) but different masses (hence different neutrons) • Both protons and neutrons have a mass of 1 • Electron’s mass is not taken into account in the mass of an atom • Ions are anything with an electrical charge • Positively charged ions have lost electrons • Negatively charged ions have gained electrons Question #4 • A scientist comparing K and Ca would find that K has a— • A: lower electronegativity and a smaller atomic radius • B: higher electronegativity and a smaller atomic radius • C: lower electronegativity and a larger radius • D: higher electronegativity and a larger atomic radius Answer to Q#4 • Letter C: lower electronegativity and a larger atomic radius • Why? Electronegativity gets higher as you go across a period and atomic radius gets smaller as you go across a period • K is farther left so it must have a lower electronegativity and larger atomic radius Question #5 • Which of the following elements is the most reactive? • A: Cs • B: Sr • C: Mg • D: Rb Answer to Q#5 • Letter A: Cs • Why? All these elements are metals—the most reactive metals are the largest • Atomic radius gets larger as you go down a group • Cs is farthest down so it is the largest and most reactive Trends Quick Review • Trends as you go down a group: larger atomic radius, lower electronegativity, lower and ionization energy (easier to lose an electron) • Trends as you go across a period: smaller atomic radius, higher electronegativity, and higher ionization energy (harder to lose an electron) • Remember IE is the energy it takes to lose an electron • Electronegativity is the ability to gain an electron Quick Review Continued • Metal reactivity: Largest metals = most reactive metals. • Remember metals like to lose electrons • Non-metal reactivity: smallest nonmetals = most reactive non-metals. • Remember non-metals like gain electrons • Noble gases are non-reactive—they have a full valence shell Question #6 • A bond between an element of group 2 and an element of group 17 will be: • A: ionic • B: Nonpolar covalent • C: Polar covalent • D: Metallic Answer to Q#6 • Letter A: Ionic • Why? Group 2 elements are metals (also known as alkaline earth metals) and group 17 (or 7) elements are nonmetals (also known as halogens) • Metals + non-metals = ionic bonding Bonding Quick Review • Covalent bonding = sharing of electrons between non-metals • Ionic bonding = transferring of electrons between metal + non-metal • Nonpolar covalent bond = equal sharing of electrons between nonmetals • Polar covalent bond = unequal sharing of electrons between non-metals Question #7 • • • • • What is the name for Cu2S? A: Copper sulfide B: Copper (I) sulfide C: Dicopper sulfide D: Dicopper monosulfide Answer to Q#7 • Letter B: Copper (I) sulfide • Why? Copper is a transition (d block) metal, so you must use roman numerals to indicate its charge • Look behind S for the charge of Cu. • Charge is +1 so copper (I) sulfide Question #8 • Which of the following is the formula for carbonic acid? • A: HC • B: HCO • C: HO • D: H2CO3 Answer to Q#8 • Letter D: H2CO3 • Why? It’s an acid so H must come first. Carbonic = carbonate = CO3-2 • H+1 + CO3-2 = H2CO3 Question #9 • What is the molecular formula of tetraphosphorus decoxide? • A: PO • B: P4O • C: P4O10 • D: PO10 Answer to Q#9 • • • • Letter C: P4O10 Why? Tetra = 4 and deca = 10 So P4O10 Remember, when using prefixes don’t switch—simply write what’s given Naming Quick Review • Must use roman numerals to indicate charge of a transition metal • Only use prefixes when the compound is all non-metals • If an acid has hydro prefix then it’s H+element • If no hydro prefix for an acid then it’s H+polyatomic Question #10 ___C3H4 + ___O2 ___CO2 + ___H2O • When the equation above is balanced, what is the sum of the coefficients? • A: 4 • B: 5 • C: 8 • D: 10 Answer to Q10 • Letter D: 10 • Why? C3H4 + 4O2 3CO2 + 2H2O; so 1+4+3+2 = 10 • Don’t forget the law of conservation of mass says the reactants side = products side Question #11 • Which of the following is an example of a decomposition reaction? • A: 2AgCl 2Ag + Cl2 • B: CuO + H2O Cu(OH)2 • C: AgCl + Mg MgCl2 + Ag • D: HCl + Na(OH) H2O + NaCl Answer to Q #11 • Letter A: 2AgCl 2Ag + Cl2 • Why? Decomposition is when you start with one reactant and break into 2 products • Letter B = synthesis; letter C = single replacement and letter D = neutralization reaction Question #12 • 12 x 403 = • What is the answer to the above problem expressed with proper sig figs? • A: 5,000 • B: 4800 • C: 4830 • D: 4836 Answer to Q#12 • Letter B: 4800 • Why? When multiplying or dividing, you use lowest # of sig figs for your answer • 12 only has two sig figs, so your answer can only have two Question #13 • How many atoms of Na are in 0.300 moles of Na? • A: 0.0131 atoms • B: 7.86x1021 atoms • C: 1.81x1023 atoms • D: 6.02x1023 atoms Answer to Q#13 • Letter C: 1.81x1023 atoms • Why? 0.300 moles Na x 6.02x1023atom 1 mole = 1.81x1023 atoms Question #14 • How many moles are in a 342grams of CaO? • A: 6.10 moles • B: 56.1 moles • C: 1.92x104 moles • D: 3.67x1024 moles Answer to Q#14 • Letter A: 6.10 grams • Why? 342g x 1mole CaO = 6.10 moles 56.08 grams • 56.08 grams is the molar mass of CaO from the Periodic Table Moles Quick Review • When multiplying/dividing use lowest # of sig figs for answer • When adding/ subtracting use lowest # of decimal places for answer • MassMoles use mass of Periodic Table = 1 mole • Moles Particles use 1 mole = 6.02x1023 particles • Moles Liters at STP use 1 mole = 22.4 L Question #15 • • • • • Be + 2HCl BeCl2 + H2 Using the above reaction, what mass of beryllium was consumed in the reaction if 4.0 moles of HCl were used? A: 2.0grams B: 9.0 grams C: 18.0 grams D: 36.0 grams Answer to Q#15 • Letter C: 18.0 grams • Why? • 4.0molsHCl x 1mols Be x 9.01gBe 2 mols HCl 1 mole Be = 18.0 grams Be Letter #16 • • • • • 2C2H6 + 7O2 4CO2 + 6H2O In an experiment, 0.500 mols of C2H6 were reacted with 1.50 moles of oxygen gas. Which of the following is the limiting reactant in this experiment? A: C2H6 B: O2 C: CO2 D: H2O Answer to Q #16 • Letter B: O2 • Why? 0.500molsC2H6 x 4mols CO2 2 mols C2H6 = 1.00 mols CO2 • 1.50molsO2x4mols CO2 = 0.857molsCO2 7 mols O2 • Since O2 produces less CO2, it must be the limiting reactant • C and D shouldn’t even be choices b/c they are products! Stoich Quick Review • Mass A Mols A Mols B Mass B • Go from mols A mols B using mol to mol ratio from coefficients of balanced equation • Limiting reactant is the one used up first. It also produces the smaller amount of product • % yield = (actual/theoretical) x100 • Can also use stoich to go from mass/mols of A to liters of B; use 22.4 L = 1mol at STP Question #17 • Which of the following is NOT a part of the kinetic molecular theory? • A: Gases move in a straight, continual motion • B: Gases have no volume themselves • C: Gases participate in inelastic collisions • D: Kinetic energy is directly related to temperature • E: Gases feel no attractive forces Answer to Q#17 • Letter C: Gases participate in inelastic collisions • Why? Gases actually participate in elastic collisions. • The other 4 parts are all TRUE Gas Laws Quiz Review • Boyle’s Law P1V1 = P2V2, where P and V are inversely related. • Charles’ Law V1/T1 = V2/T2 • Gay-Lusac P1/T1 = P2/T2 • Combined Gas Law (P1V1)/T1 = (P2V2)/T2 • Ideal Gas Law PV = nRT, where R = 0.0821 (L*atm)/(mol*K) More Gas Laws Review • Must always convert temperature from celsius to KELVIN! • Absolute zero is -273C or 0K! • Absolute zero is the point where all motion slows down and stops • Dalton’s Law P1+P2+P3… = Ptotal Question #18 • Which of the following phase changes occurs at the arrow? • A: Sublimation • B: Melting • C: Evaporation • D: Condensation Answer to Q#18 • Letter B: melting • Why? Graph always goes S, L, G—look at pic on right • The arrow points to line b/w solid and liquid • Only phase changes there are melting (SL) or freezing (LS) Phases Quick Review • 6 phases changes – Melting (SL) --Freezing (LS) – Evaporation (LG) --Condensation (GL) – Sublimation (SG) --Deposition (GS) • Molar heat of fusion = amount of energy needed to melt 1 mole of a substance • Molar heat of vaporization = amount of energy needed to evaporate 1 mole of a substance • Specific heat capacity = amount of energy needed to raise the temperature of 1 gram of a substance by 1˚C use q = mxCpxΔT IMF Quick Review • Four different intermolecular forces – London dispersion (weakest) = nonpolar covalent molecules – Dipole-dipole = polar molecules – Hydrogen bonding = H connected to N, O or F bonding to another O, N or F – Ionic (strongest) = metal + non-metal • Don’t forget to do the arrow test to determine polarity of a molecule Question #19 • How many moles of MgCl2 are present in 3.00 liters of a 0.150M MgCl2 solution? • A: 0.0500 moles • B: 0.450 moles • C: 1.35 moles • D: 20.0 moles Answer to Q#19 • • • • • Letter B: 0.450 moles Why? Molarity (M) = mols/liters So 0.150 M = x/3.00 L (0.150M)(3.00L) = x X = 0.450 moles Solutions Quick Review • Molarity(M)=mols of solute/L of solution • Molality(m)=mols of solute/kg of solvent • Freezing point depression: ΔTf=(-Kf) (m)(n) • Boiling point elevation ΔTB=(Kb)(m)(n) • Remember if the solute is covalent, n = 1 Equilibrium Quick Review • Write K expressions as products over reactants. Remember the coefficients in the balanced equation are used as exponents in the K expressions • Solids and liquids are not included! • Catalysts lower the activation energy of a reaction • Enthalpy (ΔH) and entropy (ΔS) determine the spontaneity of a reaction • - ΔH (exothermic) and +ΔS (more disorder) means a spontaneous reaction Question #20 • PCl5(g) + heat PCl3(g) + Cl2(g) • Which of the following will cause an increase in the equilibrium concentration of Cl2 gas? • A: The addition of PCl3 gas • B: The removal of PCl5 gas • C: A decrease in temperature • D: An increase in temperature Answer to Q#20 • Letter D: An increase in temperature • Why? Heat is a reactant, so increase reactant side, equilibrium shifts to product side • Letter A causes shift to L; letter B causes shift to L; and letter C causes shift to L Acid/Base Quick Review -log[H+] • pH = • pOH = -log [OH-] • pH + pOH = 14 • [H+][OH-] = 1x10-14 • [H+] = 10^(-pH) • [OH-] = 10^(pOH) • pH < 7 = acid • pH > 7 = base