L12-Sept.25-PracProbCh1
advertisement

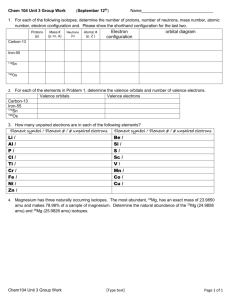
Secondary 4 – Science Name_________________ Chapter 1 – Practice Review Questions : September 25, 2014 1) Draw the Bohr-Rutherford Atomic Model for the following elements: Nitrogen and Neon 2) Draw the Lewis Structure for the following elements: Nitrogen, Neon, Argon, and Magnesium. 3) Complete the following objectives using the periodic table below. a) Label the following families: Alkali Metals, Alkaline Earth metals, halogens, and Inert /Noble gases. b) Answer each of the following questions: Which element has 27 protons in its nucleus?_____________ How many neutrons does Phosphorous have?____________ What is the family name of Calcium?________________ How many orbitals does an atom of Polonium have?______________ How many valence electrons does a bromine atom contain?______________ How many valence electrons does a fluorine atom contain?_______________ What is the atomic mass of Mg?__________________ What is the atomic number of nitrogen?___________________ How many electrons does oxygen have?__________________ Which element has more orbital’s (shells): Iodine or Cesium?__________________ 4) What is the definition for a Family or Group and what is the definition for a Period of the periodic table? Give an example of each one. 5.) Complete the following table Categories 3 examples Metals Sodium Nonmetals Hydrogen Metalloids Silicon Name 3 characteristics of each category 6) Give a definition for a valence electron. Draw a picture of any atom and label the valence electron(s) 7) a) Convert 2500 grams into kilograms. b) Convert 350 grams into kilograms. Isotopes 8) For each of the following isotopes, write the number of protons, neutrons, and electrons. Carbon12 Carbon16 Chromium- Chromium58 63 # of protons # of protons # of neutrons # of neutrons # of electrons # of electrons 9) Magnesium has three isotopes, Mg−24, Mg−25, and Mg−26. The percentage of each in order is 78.70%, 10.13%, 11.17%. Calculate the relative atomic mass of Magnesium. Application question: 10) Antimony (Atomic weight 121.75) has two naturally-occurring isotopes with isotopic weights 120.9038 and 122.9041. What is the percentage abundance of the heavier isotope? Moles 11) How many moles are in 25 grams of water ? 12) How many grams are in 4.5 moles of Li2O ? If you are finished and would like to practice more problems try the following: p. 32 of the textbook # 28, 29, 30, 31,32,34