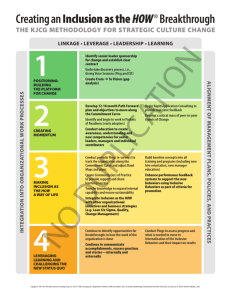
Document
advertisement

Industrially Scaleable Process for the Purification and Refolding of Inclusion Body Recombinant Protein BELINDA C. CLARKE1, GARETH M. FORDE1, CHARLES HARDY2, ALEC DREW2, ROBYN O’HEHIR2 1: Department of Chemical Engineering, Melbourne, Victoria, 3800, Australia. 2: CRC for Asthma, Dept. of Immunology, Central and Eastern Clinical School, Monash University, The Problem The Objective There is a large and increasing demand for the production of recombinant protein for use in pre-clinical, clinical and commercial applications. Traditionally, inclusion body recombinant protein is manufactured using complicated laboratory scale unit operations. However, the biotechnology industry is maturing and patent protection periods are beginning to expire. This is driving a global shift towards higher productivity, commercially viable processes. The aim of this research is to develop, optimise and analyse a scaleable, commercially viable process for the production of recombinant protein from inclusion bodies. Initially, this process will be used to produce a latex allergen (Hev b 6) for pre-clinical studies at the CRC for Asthma. The protein needs to be of high purity and concentration (i.e. no endotoxins), biologically active, and in a refolded formed. What is an inclusion body? When E.coli is transformed to manufacture large amounts of recombinant protein, the protein sometimes forms dense aggregates of insoluble misfolded proteins, known as inclusion bodies. The benefit from a production aspect of inclusion bodies is that they allow high protein concentrations, protect sensitive proteins from proteolytic (enzymatic) degradation and protect the cell from any toxic proteins. However, the challenge is to solubilise and refold this protein into its correct ‘active’ form. 1. Fed Batch Fermentation 6. Refolding Vessel The Process Cell lines transformed with the appropriate DNA supplied by the CRC for Asthma are grown and induced to express Hev b 6. The protein is renatured by decreasing the concentration of the denaturant urea. This step will be performed on the adsorption column to help prevent aggregation. 2. Chemical Disruption and Extraction 5. Expanded Bed Adsorption Column The cells are lysed to release the inclusion bodies and the protein is denatured using a chemical wash containing urea. Spermine is also added to reduce the negative effects of DNA on the adsorption column efficiency. The protein is purified using an automated chromatography unit (Biorad DuoflowTM) via an affinity agarose adsorbent (Ni charged resin) which has specificity for the His-tagged Hev b 6 protein. 3. Conditioning CaCl2 is added to remove the EDTA, as EDTA decreases the efficiency of the adsorption column. http://web.mit.edu/king-lab/www/research/Scott/Scott-Research.html 4. Filtration Removes precipitates and the DNA-spermine complexes. Some concentration of the feedstock will also occur. Preliminary Results Protein Refolding Chromatographic purification (2 ml packed bed) of the Hev b 6 protein has been performed as an analytical method to facilitate optimisation studies of protein expression and to bench-mark against the original CRC for Asthma protocol. These results will determine optimal operating parameters (e.g. expression time, induction cell density, inducer concentration) for the scaleable protein production process. The most significant challenge of this process is the refolding step as; ▪ Small amounts of contaminants can decrease the yield of refolded protein (for example, contaminants can cause aggregation and proteases cause degradation of the protein). ▪ The protein must be in an environment that favours the formation of the correctly folded proteins over the misfolded. This may require chaperones, metal ions etc. ▪ Refolding conditions vary with proteins. Chromatograph for Purification of Hev b 6-His Tagged Protein Indication of protein concentration leaving column Fractions collected and analysed using Bradford Assay and SDS-Page to identify the products Bound protein eluted (total of approximately 3.8 mg protein from Bradford Assay) Acknowledgement: