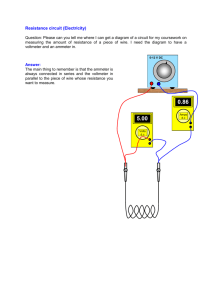
Electrical energy VI t
advertisement
Joule’s Law (or Electrical Equivalent of Heat) THEORY: When and electric heating coil is immersed in water, electrical energy is converted into heat energy. Electrical energy is usually measured in joules, whereas heat energy is frequently measured in kilocalories. The constant, J, is the conversion factor between joules and kilocalories, and is historically known as the equivalent of heat. It is important because it tells how many joules of energy is equivalent to one kilocalorie of heat. The amount of energy used in an electrical circuit is given by E = VIt, where V is voltage in volts, I is current in amps, and t is time in seconds. E is, of course, in Joules. The amount of heat energy received by water from any source can be calculated from: H = (mwCw + mcCc)(T2 – T1) Where m is mass, w is water, C is specific heat, c is calorimeter, and T is temperature. The equivalent of heat can be calculated from: J = Electrical energy Heat energy VIt . (mwCw + mcCc)(T2 – T1) Accepted value of J = 4186 Joules/kilocalorie PROCEDURE: 1. Mass the empty calorimeter cup and record its mass in kg on the data page. Fill the calorimeter to within two centimeters of the top and re-mass, recording this mass in kg on the data page. 2. Place the immersion heater into the water in the calorimeter, making sure that the coils are entirely covered with water (why?) 3. Connect the immersion heater to the power supply and to an ammeter in series. Set the ammeter to the 10-amp scale. Connect a voltmeter in parallel across the immersion heater coils. Set the voltmeter to the 200-volt scale. Have your circuit approved by the instructor before turning anything on. 4. Insert the temperature probe into the water and record the initial temperature. This will be the temperature at time t = 0.00 seconds. 5. Turn on the power supply and increase the current until it reads bout 2 amps on the ammeter. At the same time, start the graphing function on the Labquest and begin stirring the water vigorously. Record the current in amps and the voltage in volts. 6. After the temperature has risen to about 60, take the final data and stop the graphing function. 7. If possible, print out the graph. 8. Repeat steps 4 through 7 with new cold water using currents of three amps and four amps. Analysis: 1. Discuss the shape of your graph and what it tells you. Discuss some of the reasons it may not be perfectly linear. 2. Calculate the electrical equivalent of heat, J, for each run. Compare each value with the accepted value of 4189 J/kCal. Joule’s Law Data Page RUN 1: mc, mass of calorimeter ________________________kg mc+w, mass of calorimeter and water_______________kg mw, mass of water alone _________________kg current ________________amps voltage _______________volts initial temperature _________________C final temperature __________________C RUN 2: mc, mass of calorimeter ________________________kg mc+w, mass of calorimeter and water_______________kg mw, mass of water alone _________________kg current ________________amps voltage _______________volts initial temperature _________________C final temperature __________________C RUN 3: mc, mass of calorimeter ________________________kg mc+w, mass of calorimeter and water_______________kg mw, mass of water alone _________________kg current ________________amps voltage _______________volts initial temperature _________________C final temperature __________________C