Gas Laws - Walton High
advertisement

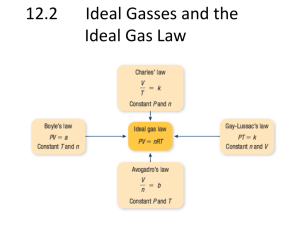
Gas Laws Properties of Gases Have mass Have low densities Easily compressed Completely fill their container Can move through each other rapidly Diffusion: the movement of one substance through another Exert pressure Gas Pressure Caused by collisions of the gas molecules with the sides of their container Pressure depends on temperature Directly proportional: will both go up or go down simultaneously Kinetic-Molecular Model Gas particles are: Constant, random, straight line motion Space between gas particles is large Negligible volume No forces of attraction Elastic collisions Gas Variables Temp (determines average kinetic energy of the particles) must be expressed in Kelvin Volume (usually in liters) Kelvin = °C + 273 1 cm3 = 1 mL Pressure (force exerted by particles per unit area) Gas Pressure P = F/A, where P = pressure, F = force of the gas particles, and A = area Is dependent on number of collisions of gas particles in a given time Units include Pa (pascals – SI units), atm (atmospheres), torr, mm Hg, bars, and psi Gas Pressure 1 atm is defined as the force the air in the atmosphere exerts on the Earth at sea level Conversions: 1 atm = 760 mm Hg 1 atm = 760 torr 1 atm = 101,325 Pa 1 atm = 101.325 kPa Air Pressure STP and Avogadro’s Principle STP Stands for “standard temperature and pressure” Equals 1.00 atm at 0.00 °C Avogadro’s Principle States that equivalent volumes of gases under the same conditions have an equal number of particles 1 mole of any gas at STP has a volume of 22.4 L Boyle’s Law The pressure and volume of a sample of gas at constant temp are inversely proportional to each other P1V1 = P2V2 Check this out Solving Gas Problems Using Boyle's Law A sample of helium gas in a balloon has a pressure of 240 kPa in a 1.4-L container. What will be the new pressure if the gas sample is transferred to a 3.0-L container? Step 1. Analyze the problem. Known Variables P1 = 240 kPa V1 = 1.4 L Unknown Variable P2 = ? kPa V2 = 3.0 L Step 2. Solve for the unknown. Divide both sides of the equation for Boyle's law by V2 to solve for P2. P2 = P1V1 V2 Substitute the known values into the rearranged equation. P2 = 240 kPa ( 1.4 L ) (3.0 L) Multiply and divide numbers and units to solve for P2. P2 = 240 kPa ( 1.4 L) = 110 kPa (3.0 L) Step 3. Evaluate the answer. Graph depicting change in pressure with change in volume of a gas (a) A plot of V versus P for a gas sample is a hyperbola, but a plot of V versus 1/P is a straight line (b). Sample Problem A gas occupies a volume of 2.45 L at a pressure of 1.03 atm and a temperature of 293 K. What volume will the gas occupy if the pressure changes to 0.980 atm and the temperature remains unchanged? Sample Problem The gas in a balloon has a volume of 4.0 L at 100. kPa. The balloon is released into the atmosphere, and the pressure decreases to 50. kPa. What is the new volume of the balloon? Charles’s Law At constant pressure, the volume of a fixed amount of gas is directly proportional to its absolute temperature V1 = V2 T1 T2 V1T2 = V2T1 Check this out Charles’s Law Charles’s Law Absolute Zero Predicted by Charles’s Law -273.15 °C = 0 K The hypothetical temperature at which all motion stops It cannot be attained by gases because the gas will change to a liquid before it reaches this temperature Sample Problem A gas at 27 °C occupies 6.50 dm3. What will its volume be at -23 °C? Sample Problem A gas occupies 0.105 dm3 at -173 °C. At what Celsius temperature will its volume be 0.140 dm3? Assume that pressure is constant. Gay-Lussac’s Law The pressure of a gas is directly proportional to the temperature if the volume remains constant P1 = P2 T1 T2 Check this out Gay-Lussac’s Law Sample Problem What is the pressure of a fixed volume of a gas at 30.0 °C if it has a pressure of 1.1 atm at 15.0 °C? Sample Problem What is the temperature of a fixed volume of a gas at 0.96 atm if it has a pressure of 1.0 atm at 37 °C? Combined Gas Laws Combines Charles’s, Boyle’s and Gay-Lussac’s Laws P1V1 = P2V2 T1 T2 How can you solve gas problems using the combined gas law? A gas at 100.0 kPa and 25.0°C fills a flexible container with an initial volume of 2.00 L. If the temperature is raised to 60.0° and the pressure increased to 320.0 kPa, what is the new volume? Step 1. Analyze the problem. Known Variables P1 = 100.0 k Pa P2 = 320.0 kPa T2 = 60.0°C T1 = 25.0°C V1 = 2.00 L Unknown Variable V2 = ? L Step 2. Solve for the unknown. Add 273 to the Celsius temperature for T1 and T2 to obtain the kelvin temperature. T1= 273 + 25.0°C = 298 K; T2 = 273 + 60.0°C = 333 K Multiply both sides of the equation for the combined law by T2 and divide by P2 to solve for V2. P1V1 = P2 V2 T1 T2 V2 = V1(P1)(T2) (P2)(T1) Substitute the known values into the rearranged equation; multiply and divide numbers and units to solve for V2. V2 = 2.00 L ( 100.0 kPa)( 333 K) = 0.698 L (320.0 kPa) (298 K) Step 3. Evaluate the answer. Sample Problem A sample of oxygen gas has a volume of 7.84 cm3 at a pressure of 71.8 kPa and a temperature of 25.0 °C. What will be the volume of the gas if the pressure is changed to 101 kPa and the temperature is changed to 0.0 °C? Sample Problem The volume of a sample of gas is 200. mL at 275 K and 92.1 kPa. What will the new pressure be if the gas occupies 238 mL at 350. K? Ideal Gas Law Describes the physical behavior of an ideal gas in terms of the pressure, volume, temperature, and the # of moles of gas There is no ideal gas, but the formula is helpful in describing the behavior of real gases. Ideal Gas Law PV = nRT R is called the gas constant, also 0.0821 atm·L/mol·K (Value of R can change based on its units) N = # of moles Solving Gas Problems Using the Ideal Gas Law Calculate the number of moles of gas contained in a 2.0-L container at 200 K with a pressure of 120 kPa. Step 1. Analyze the problem. Known Variables V = 2.0 L T = 200.0 K P = 120 kPa R = 8.314 L•kPa/(mol•K) Unknown Variable n = ? mol Step 2. Solve for the unknown. Divide both sides of the ideal gas law equation by RT to solve for n. PV = nRT n=PV RT Substitute the known values into the rearranged equation. n = (120 kPa)(2.0 L) (8.314 L•kPa)(200.0 K) (mol•K) Multiply and divide numbers and units to solve for n. n = (120 kPa)(2.0 L) = 0.14 mol (8.314 L•kPa)(200.0 K) (mol•K) Step 3. Evaluate the answer. Sample Problem How many grams of argon would it take to fill a light bulb with a volume of 0.475 L at STP? Sample Problem What is the pressure exerted by 0.625 moles of a gas in a 45.4 L container at -24.0 °C? Dalton’s Law of Partial Pressures The sum of the partial pressures of all the components in a gas mixture is equal to the total pressure of the gas mixture PT = pa + pb + pc + . . . Mole fractions can be used to determine partial pressure mole gas A x PTotal = Pa total # moles gas Dalton’s Law Sample Problem What is the atmospheric pressure if the partial pressures of nitrogen, oxygen, and argon are 77.75 kPa, 19.94 kPa, and 1.99 kPa, respectively? Sample Problem What partial pressure of oxygen is a scuba diver breathing if the total pressure is 6.3 atm, and 20. % of the air is oxygen? What is meant by “water displacement?” Sample Problem A gas is collected over water at 30. °C. The total pressure inside the apparatus is 160. kPa. What is the partial pressure of the dry gas? Graham’s Law Under the same conditions (constant temp and press), gases diffuse at a rate inversely proportional to the square roots of their densities (or molecular masses) Graham’s Law v = velocity of gas m = mass Graham’s Law Effusion - The movement of atoms or molecules through a hole so tiny that they do not stream through, but instead pass through one particle at a time Similar to diffusion Lighter gases effuse and diffuse faster than heavier gases because the particles of the lighter gas move faster than the particles of the heavier gas. Graham’s Law Sample Problem What is the relative rate of diffusion between hydrogen and oxygen gases? Sample Problem A molecule of oxygen gas travels about 250. m/s at 0 °C. How fast would an atom of xenon travel at the same temperature? Sample Problem The rate of diffusion of an unknown gas is 4.00 times faster than oxygen gas. Calculate the molar mass of the unknown gas.