Lecture 6: Intro to Entropy
advertisement

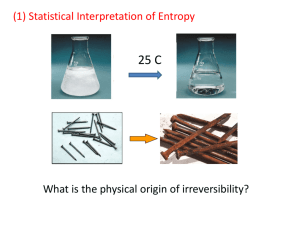
Lecture 5: Intro to Entropy • Reading: Zumdahl 10.1, 10.3 • Outline: – Why enthalpy is not enough – Statistical interpretation of entropy – Boltzmann’s Formula Enthalpy and Spontaneous Rxns • Early on in the development of thermodynamics, it was believed that if a reaction was exothermic, it was spontaneous. • Consider the following reaction: H2O(s) H2O(l) DH°rxn = +6.02 kJ • Endothermic…..yet spontaneous! Enthalpy and Spontaneous Rxns • Consider the following problem: Mixing of a gas inside a bulb adiabatically (q = 0). • q = 0, w = 0, DE = 0, and DH = 0 ….but it still happens Statistical Interpretation of Entropy • Imagine that we have a collection of 3 distinguishable particles who have a total energy of 3e. • Let’s ask the question, “How will this fixed amount of energy distribute itself over the particles?” Statistics and Entropy (cont.) • Our system consists of three distinguishable particles. quanta 3 2 1 1 2 3 • There are three “quanta” of energy (e) available for a total of energy of “3e” First Arrangement: All on one • The first possible arrangement we consider is one in which all energy resides on one particle 3 3 3 2 2 2 1 1 1 1 2 3 1 2 3 There are three ways to do this 1 2 3 Second Arrangement: 2, 1, 0 • Next arrangement: 2e on 1, 1e on another, and the third has 0e 3 3 3 2 2 2 1 1 1 1 2 1 3 2 3 3 3 3 2 2 2 1 1 1 1 2 3 1 2 3 Six ways to do this 1 2 1 2 3 3 Third Arrangement • The final possible arrangement is 1e on each particle. 3 2 1 1 2 3 Only one way to do this. Which Arrangement? 3 3 ways 2 1 1 2 • Which arrangement is most probable? 3 3 6 ways 2 1 1 2 3 • Ans: The arrangement which the greatest number of possibilities 3 1 way 2 • In this case: “2, 1, 0” 1 1 2 3 The Dominant Configuration • Configuration: a type of energy distribution. • Microstate: a specific arrangement of energy corresponding to a configuration. • Which configuration will you see? The one with the largest # of microstates. This is called the dominant configuration. Determining Weight • Weight (W): the number of microstates associated with a given configuration. • We need to determine W, without having to write down all the microstates. A! A! W W a0 !a1!... ai ! i A = the number of particles in your system. ai is the number of particles with the same amount of energy. ! = factorial, and P means take the product. Determining Weight (cont.) • Consider 300 students where 3 students have 1e of energy, and the other 297 have none. • A = 300 a1 = 3 a0 = 297 A! W ai! i 300! 3!297! = 4.5 x 106 Weight and Entropy • The connection between weight (W) and entropy (S) is given by Boltzmann’s Formula: S = k lnW k = Boltzmann’s constant = R/Na = 1.38 x 10-23 J/K • The dominant configuration will have the largest W; therefore, S is greatest for this configuration Example: Crystal of CO • Consider the depiction of crystalline CO. There are two possible arrangements for each CO molecule. • Each arrangement of CO is possible. • For a mole of CO: W = Na!/(Na/2!)2 = 2Na Example: Crystal of CO • For a mole of CO: W = Na!/(Na/2!)2 = 2Na • Then, S = k ln(W) = k ln (2Na) = Nak ln(2) = R ln(2) = 5.64 J/mol.K Another Example: Expansion • What is DS for the expansion of an ideal gas from V1 to 2V1? • Focus on an individual particle. After expansion, each particle will have twice the number of positions available. Expansion (cont.) • Original Weight = W • Final Weight = 2W • Then DS = S2 -S1 = k ln(2W) - kln(W) = k ln(2W/W) = k ln(2) Expansion (cont.) • Therefore, the DS per particle = k ln (2) • For a mole of particles: DS = k ln (2Na) = Nak ln(2) = R ln(2) = 5.64 J/mol.K Expansion (cont.) • Note in the previous example that weight was directly proportional to volume. • Generalizing: DS = k ln (Wfinal) - kln(Winitial) = k ln(Wfinal/Winitial) = k ln(Wfinal/Winitial) = Nk ln(Wfinal/Winitial) for N molec. = Nkln(Vfinal/Vinitial)