AP Chemistry - My Teacher Pages
advertisement

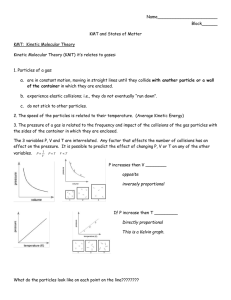
Introduction to Gases & The Kinetic Molecular Theory (KMT) Note: The content in this presentation is not required in the IB course syllabus for topic 1 but will help you to deepen your understanding of the gas laws and help you later in Kinetics. Learning Objective from Chapter 6 (Kinetics) 6.2.1 - Describe the kinetic theory in terms of the movement of particles whose average energy is proportional to temperature in kelvins. The Kinetic Molecular Theory of Gases This theory tries to model gas particles themselves at a microscopic level and consists of 5 main postulates. 1. Gases are composed of very small particles, either molecules or individual atoms. 2. The gas particles are tiny compared to the distances between them, so we assume that the volume of the gas particles themselves is negligible. 3. These gas particles are in constant motion, moving in straight lines in a random fashion and colliding with each other and the inside walls of the container. The collisions with the inside container walls are what comprise the pressure of the gas. Q - What would increasing the number of gas particles do to the number of collisions (pressure)? Check it! Q - What would increasing the size of the box do to the number of collisions (pressure)? Check it! Q - What would increasing the temperature of the gas particles do to the number of collisions (pressure)? Why? Check it! Balloons and Tires and Sodas 1. What happens when you add additional air to a balloon? When you add additional air to your bicycle tire? 2. What happens to a balloon when you squeeze it? 3. What happens to a helium balloon when you place it in your car on a cold winter day? on a hot summer day? 4. What happens to your tire pressure on a cold winter morning? on a hot summer day? 5. What happens to soda when you shake it up before opening it? What happens after you open the soda? Factors influencing gases: P, V, T, n Learning Objective from Chapter 6 (Kinetics) 6.2.5 - Sketch and explain qualitatively the Maxwell–Boltzmann energy distribution curve for a fixed amount of gas at different temperatures and its consequences for changes in reaction rate. The Maxwell–Boltzmann distribution Go back to describes particle speeds in gases, where the simulation particles do not constantly interact with each other but move freely between short collisions. The speed probability density functions of the speeds of a few noble gases at a temperature of 298.15 K (25 °C). The y-axis is in s/m so that the area under any section of the curve (which represents the probability of the speed being in that range) is dimensionless. …now back to the kinetic molecular theory… 4. The gas particles are assumed to neither attract nor repel each other. They may collide with each other, but if they do the collisions are assumed to be elastic. No kinetic energy (KE) is lost, only transferred from one gas molecule to another. KE per molecule = 1/2 mv2 (m=mass of the individual molecule) The average kinetic energy of the gas is proportional to the Kelvin temperature. 5. • • KE per mol = 3/2 RT KE = 3/2 RTn or… A gas that obeys these five postulates is called an ideal gas. • • • Q - Do we live in the ideal world? A - No, because the universe hates you… but real gases, which we will discuss later do behave much like the ideal ones. Pressure Q - According to the KMT, what is pressure? A - P is the force exerted by gas molecules colliding with the walls of their container. Q - How do we measure pressure? Atmospheric Pressure - Barometer Container Pressure - Manometer Barometers Manometers Units of Pressure Be careful to note the units given in a problem! Most gas law formulas can use any pressure unit as long as you use the same one on both sides (ex. PV=PV) kPa must be used for the ideal gas law (PV=nRT) if 8.314 is used for R 1 atm = 760 mm Hg = 760 torr = 101.3kPa = 14.5psi Gases Keywords & Equations urms=root mean square speed=(3kT/m)1/2=(3RT/M)1/2 * r=rate of effusion STP=0oC & 1 atm PV=nRT PV/T=PV/T (Combined Gas Law) PA=Ptotal x XA, where XA=moles A/ total moles * Ptotal= PA + PB + PC + … (Dalton’s Law of Partial Pressures) * KE per molecule = 1/2 mv2 * KE per mol = 3/2 RTn r1/r2=(M1/M2)1/2 (Graham’s Law) * 1 atm = 760 mm Hg = 760 torr = 101.1 kPa * = probably not on IB test but still something you would get in any college chemistry class Root Mean Square Speed The third postulate can be quantified by calculating the average velocity of the gas particles. This quantity is written as urms It is the speed of a gas particle having the average kinetic energy of the all the gas particles in the container. Q - How do we measure this KE? A - A thermometer! urms=(3RT/M)1/2 R=8.3145 J/K x mol (The “Universal” gas constant) T= Temperature in K M=the molar mass of the gas For Hydrogen at room temp, urms= 2000m/s! Root Mean Square Speed urms=(3RT/M)1/2 Calculate the urms for the following gases at 20oC. Argon Carbon dioxide Oxygen Q - Which is the highest? Lowest? Why? Q - Would that change if you increased the temperature? Root Mean Square Speed urms=(3RT/M)1/2 Q - Remember that urms is an “average” speed. What does that mean? A – Some particles in the sample are going faster than urms and some are going slower.