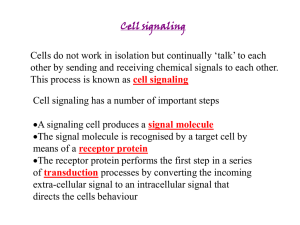
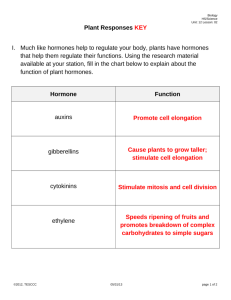
Chapter 12
advertisement

Section 5 Advanced Metabolism Chapter 16 Integration of Metabolism Section 16.1: Overview of Metabolism Anabolic and catabolic reaction pathways that use carbohydrates, lipids, and proteins as energy and biosynthetic precursors must be precisely regulated Except during youth, illness, or pregnancy, the animal’s tissues exist in a metabolic steady state (anabolic is approximately equal to catabolic) Section 16.1: Overview of Metabolism Figure 16.1 Overview of Metabolism Section 16.1: Overview of Metabolism Intracellular communication is believed to play a significant role in maintaining metabolic balance Intracellular communication occurs by means of chemical signals Once released into the extracellular environment, each chemical signal is recognized by a specific cell (target cell) In animals, the nervous and endocrine systems are responsible for controlling metabolism Section 16.1: Overview of Metabolism Figure 16.2 Structure of the Thyroid Hormones T3 and T4 Regulation by the endocrine system involves the release of hormones (endocrine hormones) from glands into the blood, which then travel to target cells An example is thyroid-stimulating hormone (TSH) that stimulates follicular cells from the thyroid to release T3 and T4 T3 stimulates glycogenolysis in the liver Section 16.2: Hormones and Intracellular Communication Peptide Hormones In mammals many metabolic activities are controlled by peptide hormones They initiate their action by binding to receptors on the outer surface of the target cell’s plasma membrane Synthesis and secretion of many of these hormones are regulated by a complex cascade controlled by the central nervous system Sensory signals are received by the hypothalamus, an area of the brain that integrates the nervous and endocrine systems Section 16.1: Overview of Metabolism Intracellular actions are often triggered by second messengers (e.g., cAMP) Second messengers often act to modulate an enzyme cascade in order to amplify the signal and response Figure 16.3 Signal Transduction Section 16.2: Hormones and Intracellular Communication Animals employ several mechanisms to prevent excess hormone synthesis and release, most notably negative feedback For example, T3 and T4 inhibit TSH release Target cells also possess a desensitization mechanism to decrease the number of receptors for the hormone Downregulation is a decrease in receptor number in response to a specific signal For example, type 2 diabetes is due to a decrease in functional insulin receptor (insulin resistance) Section 16.2: Hormones and Intracellular Communication There are two types of receptors that water-soluble hormone molecules bind to: G-protein-coupled receptors and receptor tyrosine kinases G-protein-coupled receptors (GPCRs), the largest known receptor family, are composed of seven membrane-spanning helices Figure 16.4 The G-ProteinCoupled Receptor and G Protein N-terminus has a domain for ligand binding and C-terminus has a segment for interacting with G proteins Section 16.2: Hormones and Intracellular Communication GPCRs respond to neurotransmitters as well as hormones G proteins (heterotrimeric GTP-binding proteins) are the molecular switches that transduce ligand binding to intracellular signals Figure 16.4 The G-ProteinCoupled Receptor and G Protein Composed of an a, b, and g subunit The a subunit binds GTP Section 16.2: Hormones and Intracellular Communication G proteins are attached to the membrane by myristoyl and/or palmitoyl groups The bg subunits inhibit the a subunit The bg subunit promotes association of the a subunit with the GPCR and prevents GDP/GTP exchange G-protein activation occurs when a ligand binds the GPCR Causes a conformational change leading to GDP/GTP exchange, mediated by a guanine nucleotide exchange factor (GEF) Followed by GTP-a subunit dissociation Section 16.2: Hormones and Intracellular Communication GTP-as activates adenylate cyclase while GTP-ai inhibits the enzyme Adenylate cyclase produces the second messenger cyclic AMP (cAMP) There are other second messengers: calcium ions, cGMP, and phosphatidylinositol components Figure 16.5 Structure of the Second Messenger Molecule Cyclic AMP (cAMP) Section 16.2: Hormones and Intracellular Communication cAMP—generated from ATP by adenylate cyclase in response to hormonereceptor interaction The G protein Gs stimulates cAMP production when glucagon, TSH, and epinephrine bind their receptors Figure 16.6 The Adenylate Cyclase Second Messenger System That Controls Glycogenolysis Section 16.2: Hormones and Intracellular Communication A Gs undergoes GDP/GTP exchange once the ligand binds to the receptor GTP-as subunit dissociates and activates adenylate cyclase to produce cAMP Figure 16.6 The Adenylate Cyclase Second Messenger System That Controls Glycogenolysis Section 16.2: Hormones and Intracellular Communication GTP hydrolysis mediated by GTPase activating protein (GAP) inactivates the G protein (GDP-as) Activated adenylate cyclase synthesizes a number of cAMP molecules (signal amplification) cAMP diffuses into the cytoplasm and binds and activates c-AMP-dependent protein kinase (PKA) Figure 16.6 The Adenylate Cyclase Second Messenger System That Controls Glycogenolysis Section 16.2: Hormones and Intracellular Communication PKA then phosphorylates and thereby alters the activity of key regulatory enzymes cAMP target proteins are different depending on the cell type Glucagon and epinephrine both activate glycogen degradation in the liver in cAMP-dependent fashion Figure 16.6 The Adenylate Cyclase Second Messenger System That Controls Glycogenolysis Section 16.2: Hormones and Intracellular Communication The Phosphatidylinositol Cycle, DAG, and Calcium— mediate the actions of hormones and growth factors Figure 16.7 The Phosphatidylinositol Pathway Examples include acetylcholine, TSH, vasopressin, GRH, and epinephrine Phosphatidylinositol-4,5bisphosphate (PIP2) is cleaved by phospholipase C into DAG and IP3 Section 16.2: Hormones and Intracellular Communication Hormone receptor complex activates the G protein that activates phospholipase C DAG product activates protein kinase C (PKC), which activates specific regulatory enzymes IP3 diffuses to the calcisome (SER) where it binds to a calcium channel Causing calcium release Figure 16.7 The Phosphatidylinositol Pathway Section 16.2: Hormones and Intracellular Communication cGMP—synthesized from GTP by guanylate cyclase Guanylate cyclase is activated by atrial natriuretic peptide and bacterial enterotoxin Atrial natriuretic peptide (ANF) is released by heart cells in response to increased blood volume Lowers blood pressure via vasodilation and diuresis Bacterial enterotoxin activates another type of guanylate cyclase in intestinal cells and causes diarrhea Section 16.2: Hormones and Intracellular Communication Receptor Tyrosine Kinases (RTKs) —a family of transmembrane receptors that bind ligands such as insulin and epidermal growth factor Binding of ligand to the external domain activates the tyrosine kinase domain Insulin receptor has two domains: the extracellular a and the b, which has a transmembrane domain and the tyrosine kinase domain Figure 16.8 The Insulin Receptor Section 16.2: Hormones and Intracellular Communication Figure 16.9a Simplified Model of Insulin Signaling Insulin receptor substrate 1 (IRS1) is one of the proteins phosphorylated by the insulin receptor Phosphorylated IRS1 then binds and activates proteins like phosphatidylinositol-3-kinase, which phosphorylates PIP2 PIP3 then triggers a kinase cascade, including PKB activation Section 16.2: Hormones and Intracellular Communication Figure 16.9b Simplified Model of Insulin Signaling PKB stimulates glycogen synthesis and inhibits lipolysis PKB activates mTOR, which is a central kinase sensor that integrates hormonal activity, nutrient availability, stress, and energy status This is controlled by an autoregulatory pathway Section 16.2: Hormones and Intracellular Communication Growth Factors Rigorous control of cell growth and cell division is essential for survival of multicellular organisms Growth factors (hormone-like polypeptides) and some cytokines regulate growth, proliferation, and differentiation Examples of growth factors: epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and insulin-like growth factor 1 and 2 (IGF-1 and IGF-2) Examples of cytokines: interleukins and interferons Section 16.2: Hormones and Intracellular Communication Epidermal growth factor is a mitogen (stimulator of cell division) for a large number of epithelial cells Triggers cell division of epidermal and gastrointestinal lining cells by binding TKRs Platelet-derived growth factor is secreted by blood platelets during the clotting reaction Stimulates mitosis and collagen synthesis in fibroblasts during wound healing Section 16.2: Hormones and Intracellular Communication IGF-1 and IGF-2 are polypeptides that mediate the growth-promoting action of growth hormone (GH) When GH binds to its cell surface receptor, IGF-1 and IGF-2 are the major stimulators of growth in animals In addition to stimulating cell division, IGF-1 and IGF2 promote (to a lesser degree) the same metabolic processes as insulin Section 16.2: Hormones and Intracellular Communication Interleukin 2 (IL-2) promotes cell growth and differentiation and regulates the immune system Bind to activated T cells to make numerous identical T cells Interferons act as growth inhibitors and are produced by a variety of cells in response to antigen, mitogens, viral infection, and certain tumors Type I interferons protect cells from viral infections by phosphorylating and inactivating a protein necessary for protein synthesis (eIF2a) Type II are produced by T lymphocytes and inhibit the growth of cancer cells Section 16.2: Hormones and Intracellular Communication Steroid and Thyroid Hormone Mechanisms Signal transduction pathways of the hydrophobic steroid and thyroid hormones result in changes in gene expression Hydrophobic hormone molecules are transported in the blood by attaching to transport proteins (e.g., albumin and sex hormone-binding protein) Upon reaching their targets, they can diffuse through the plasma membrane and bind intracellular receptors Receptor-ligand complex migrates to the nucleus as a homodimer binds to a hormone response element and activates transcription Section 16.2: Hormones and Intracellular Communication Figure 16.10 Model of Steroid Hormone Action Within a Target Cell Section 16.3: Metabolism in the Mammalian Body: Division of Labor Each organ in the mammalian body contributes to the individual’s function in several ways: Consumers of energy or supplying energy-rich nutrients Signal molecules offer an important control mechanism for integrating these processes Nutrient transport across cell plasma membranes is also an important feature of organ function Section 16.3: Metabolism in the Mammalian Body: Division of Labor Gastrointestinal Tract Most obvious role is digestion of carbohydrate, lipids, and proteins into molecules small enough for absorption Enterocytes need large amounts of energy for active transport and lipoprotein synthesis The GI tract also produces hormones that stimulate appetite (e.g., ghrelin) or promote satiety (e.g., cholecystokinin and leptin) Insulin secretion from pancreatic b-cells Section 16.3: Metabolism in the Mammalian Body: Division of Labor Liver Performs a stunning variety of metabolic activities Key roles in carbohydrate, lipid, amino acid metabolism, and blood glucose levels Also detoxification and reduces fluctuations in nutrient availability Section 16.3: Metabolism in the Mammalian Body: Division of Labor Muscle Skeletal muscle constitutes about one-half of the body’s mass and therefore consumes a large fraction of generated energy Cardiac muscle requires glucose in the fed state and fatty acids in the fasting state Insulin activates glucose transport into skeletal and cardiac muscle through GLUT4 translocation Section 16.3: Metabolism in the Mammalian Body: Division of Labor Adipose Tissue The role of adipose tissue is primarily the storage of energy in the form of triacylglycerols Lipid metabolism is controlled by the hormones insulin and epinephrine Adipocytes and macrophages within adipose tissue secrete peptide hormones (adipokines) Leptin is a satiety-inducing protein Section 16.3: Metabolism in the Mammalian Body: Division of Labor Brain Ultimately directs most metabolic processes in the body Sensory information is integrated in several areas in the brain, which then direct activities The hypothalamus plays a pivotal role in energy balance The brain uses glucose as its sole fuel and uses up to 20% of the body’s energy resources Section 16.3: Metabolism in the Mammalian Body: Division of Labor Kidney Several roles significant to maintaining a stable internal environment: 1. Filtration of blood plasma 2. Reabsorption of electrolytes 3. Regulation of blood pH 4. Regulation of the body’s water content Section 16.4: The Feeding-Fasting Cycle Despite their constant requirements for energy, mammals only consume food intermittently This is possible because of the elaborate mechanism for storing and mobilizing energy-rich molecules derived from food Section 16.4: The Feeding-Fasting Cycle Animals must have metabolic integration and the regulatory influence of hormones Substrate concentrations are also an important factor in metabolism Figure 16.11 Nutrient Metabolism in Mammals Section 16.4: The Feeding-Fasting Cycle Figure 16.11 Nutrient Metabolism in Mammals Postprandial state is after a meal when nutrient levels are high, while postabsorptive is after an overnight fast when nutrient levels are low Section 16.4: The Feeding-Fasting Cycle The Feeding Phase The feeding phase involves food movement, digestion, and absorption into the blood and lymph in the gastrointestinal tract This is controlled by the enzyme-producing cells of the digestive organs, the nervous system, and several hormones Smooth muscle contraction propels the food along the tract and is controlled by sympathetic and parasympathetic nerves Hormones such as gastrin, secretin, and cholecystokinin (CCK) also contribute to the digestive process Section 16.4: The Feeding-Fasting Cycle In the early postprandial state, sugars and amino acids are absorbed and transported in the portal blood to the liver Most lipid molecules are transported in the lymph as chylomicrons Chylomicrons pass to the bloodstream, which provides triacylglycerol to muscle and adipose tissue Figure 16.12 The Early Postprandial State Section 16.4: The Feeding-Fasting Cycle Chylomicron remnants then deliver the phospholipids, cholesterol, and few remaining triacylglycerol molecules to the liver Cholesterol is used to make bile acids, and fatty acids are used to synthesize phospholipids The phospholipids, lipids, and protein are then incorporated into lipoproteins for export to tissues Figure 16.12 The Early Postprandial State Section 16.4: The Feeding-Fasting Cycle Glucose movement from the small intestine to the liver stimulates b cells in the pancreas to release insulin Insulin release triggers glucose uptake, glycogenesis, fat synthesis and storage, and gluconeogenesis and generally stimulates protein synthesis Substrate supply and allosteric effectors can also affect these processes Section 16.4: The Feeding-Fasting Cycle The Fasting Phase Glucagon is released as glucose and insulin levels fall back to normal Prevents hypoglycemia by stimulating glycogenolysis and gluconeogenesis in the liver Figure 16.13 The Early Postabsorptive State Section 16.4: The Feeding-Fasting Cycle If a fast becomes prolonged (e.g., overnight), maintenance of blood glucose levels occurs by fatty acid mobilization Alternative to glucose for muscle, conserving glucose for the brain Figure 16.13 The Early Postabsorptive State Section 16.4: The Feeding-Fasting Cycle Extraordinarily long fasting (starvation) leads to metabolic changes to ensure adequate glucose availability for glucose-requiring cells (e.g., brain) Additional fatty acids from adipose tissue and ketone bodies from the liver are mobilized Section 16.4: The Feeding-Fasting Cycle Glycogen is depleted after several hours, so gluconeogenesis plays an important role Large amounts of amino acids from muscle protein are used for this purpose After several weeks, the brain becomes adapted to using ketone bodies as an energy source Section 16.4: The Feeding-Fasting Cycle Feeding Behavior Regulating feeding behavior involves hormone and neuronal signals as well as sensory input from the environment (i.e., the five senses) Both are integrated in the brain to regulate appetite Figure 16.14 Feeding Behavior in Humans Section 16.4: The Feeding-Fasting Cycle The primary neural circuits controlling appetite are in the hypothalamus Primary neurons are in the arcuate nucleus (ARC) Depending on the signal (peptide hormone) produced by the ARC, it can lead to appetite suppression or stimulation Figure 16.15 Apetite-Regulating Neurons in the Arcuate Nucleus (ARC) Section 16.4: The Feeding-Fasting Cycle Insulin also reduces intake via the same neurons that the hypothalamus uses AMPK seems to mediate the appetite-regulating integration of the hypothalamus AMPK is inhibited by insulin and leptin binding to their receptors Figure 16.15 Apetite-Regulating Neurons in the Arcuate Nucleus (ARC) mTOR also regulates ARC nutrient-sensing neurons