water molecules are
advertisement

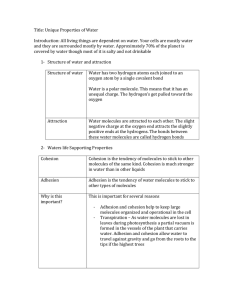
1 < 0o C - ice; 0o C - 100o C – liquid; > 100o C - steam 2 • Water consists of an Water is a Polar Molecule oxygen atom bound to -has oppositely charged two hydrogen atoms ends by two single covalent bonds. – Oxygen has unpaired & paired electrons which gives it a slightly negative charge while Hydrogen has no unpaired electrons and shares all others with Oxygen – Leaves molecule with positively and negative charged 3 ends Water molecules form Hydrogen bonds slightly positive charge hydrogen bond between (+) and (-) areas of different water molecules slightly negative charge 4 4 Water’s Properties • • • • • • • • Cohesion Adhesion Capillarity High Specific Heat High Heat of Vaporization Solid water (ice) is less dense than liquid Solvent Transparent 5 Cohesion • Water clings to polar molecules through hydrogen bonding – Cohesion refers to attraction to other water molecules. responsible for surface tension a measure of the force necessary to stretch or break the surface of a liquid 6 Adhesion – Adhesion refers to attraction to other substances. Water is adhesive to any substance with which it can form hydrogen bonds. 7 Capillary action water evaporates from leaves = transpiration adhesion, cohesion and capillary action water taken up by roots 8 •trees have specialized structures to transport water: xylem and phloem “plumbing” • water molecules are “dragged” from the roots to the top of the tree by capillary action and cohesion: hydrogen bonds help water molecules stick to each other 9 High Specific Heat – High specific heat Amount of heat that must be absorbed or expended to change the temperature of 1g of a substance 1o C. 10 Impact of water’s high specific heat ranges from the level of the whole environment of Earth to that of individual organisms. • • • • A large body of water can absorb a large amount of heat from the sun in daytime and during the summer, while warming only a few degrees. At night and during the winter, the warm water will warm cooler air. Therefore, ocean temperatures and coastal land areas have more stable temperatures than inland areas. The water that dominates the composition of biological organisms moderates changes in temperature better than if composed of a liquid with a lower specific heat. The Earth is over 75% water! 11 High Heat of Vaporization – High heat of vaporization Amount of energy required to change 1g of liquid water into a gas (586 calories). large number of hydrogen bonds broken when heat energy is applied 12 • • • As a liquid evaporates, the surface of the liquid that remains behind cools - Evaporative cooling. Evaporative cooling moderates temperature in lakes and ponds and prevents terrestrial organisms from overheating. Evaporation of water from the leaves of plants or the skin of animals removes excess heat. 13 “Universal” Solvent • • • • A liquid that is a completely homogeneous mixture of two or more substances is called a solution. – A sugar cube in a glass of water will eventually dissolve to form a uniform mixture of sugar and water. The dissolving agent is the solvent and the substance that is dissolved is the solute. – In our example, water is the solvent and sugar the solute. In an aqueous solution, water is the solvent. Water is not really a universal solvent, but it is very versatile because of the polarity of water 14 molecules. • Water is an effective solvent as it can form hydrogen bonds. – Water clings to polar molecules causing them to be soluble in water. Hydrophilic attracted to water – Water tends to exclude nonpolar molecules. Hydrophobic repelled by water 15 • Water transports molecules dissolved in it – Blood, a water-based solution, transports molecules of nutrients and wastes organisms – Nutrients dissolved in water get transported through plants – Unicellular organisms that live in water absorb needed dissolved substances 16 Solid water (ice) is less dense than liquid • Ice is less dense than water: the molecules are spread out to their maximum distance Density = mass/volume same mass but a larger volume 17 Oceans and lakes don’t freeze solid because ice floats water expands as it solidifies water reaches maximum density at 4-degrees C water freezes from the top down organisms can still live in the water underneath the ice during winter 18 Water is Transparent • The fact that water is clear allows light to pass through it – Aquatic plants can receive sunlight – Light can pass through the eyeball to receptor cells in the back 19 20 pH • • • Water ionizes into H+ and OHH 2O H+ and OHpH scale expresses hydrogen ion (H+) concentration in a solution. – logarithmic scale ranging from 0-14 neutral = 7 Below 7 = acid Above 7 = base o Water at 25 C contains 1/10,000,000 mole of H+ ions = 10 -7 moles/liter pH = -log [H+] 21 pH 22 Acids • Acids dissociate in water to increase the concentration of H+. – Have many H+ ions – Sour taste – HCl is hydrochloric acid or stomach acid 23 Bases • Bases combine with H+ ions when dissolved in water, thus decreasing H+ concentration. – Have many OH- (hydroxide) ions – Bitter taste – NaOH = sodium hydroxide or baking soda 24 Buffers • Buffers – act as a reservoir for hydrogen ions, donating or removing them from solution as necessary – Offer protection from extreme pH levels – Produced naturally by organisms: Organisms can’t tolerate much pH change Cells function best within a narrow pH range 25