Do Now

Do Now
Please update your table of contents.
Page #
96-97
Title
Ionic Bonding
Please take the sheet from the front cart and place it on pg. 97
Divide pg. 96 horizontally about ¾ down the page.
Ions on page
What happens when an atom loses an electron?
What is its new charge?
What happens when an atom gains an electron?
What is its new charge?
What happens once these ions have formed?
What holds an ionic bond together?
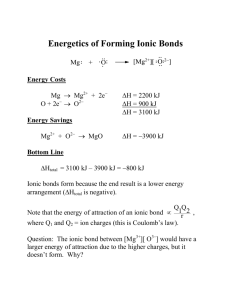
Ionic Bonds
Ionic bonds form as a result of the attraction between positive and negative ions!
Ionic Bonds
Let’s check out two videos on ionic bonding…
NOVA: How Elements Form Compounds
What are ionic bonds?
How do you know the formula for an ionic compound?
Drop and Swap
1.
2.
3.
4.
Find the charge of the ions and remove the + and – signs.
If the numbers are the same, they cancel each other out.
If the numbers are different, “drop” them and
“swap” them.
If a number is dropped to a polyatomic ion, then brackets need to be inserted.
Mg +2 + O -2
Mg
2
+ O
2
Drop and Swap
Mg +2 + Cl -1
Mg
2
+ Cl
1
Drop and Swap
Mg +2 + OH -1
Mg
2
+ OH
1
Mg(OH)
2
Magnesium hydroxide
Milk of magnesia
(NH
4
) +1 + S +2
(NH
4
)
1
+ S
2
(NH
4
)
2
S
Ammonium sulfide
Main ingredient of stink bombs
Bond With A Classmate
Find a classmate who is representing an ion with the opposite charge as your ion.
Work together to follow the steps of the drop and swap, and write the formula for the compound on your worksheet.
Once you have bonded with as many classmates as you are able, start on the Ionic Bonding Worksheet.