How many oxygen atoms are present in MgSO4 • 7H2O?
advertisement

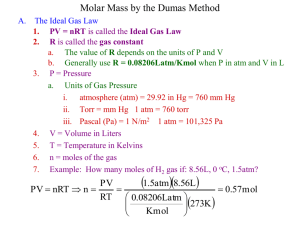
Chapter 12 Gases What is the pressure of the gas in the bulb? 1. 2. 3. 4. 5. Pgas = Ph Pgas = Patm Pgas = Ph + Patm Pgas = Ph - Patm Pgas = Patm - Ph What is the pressure of the gas in the bulb? 1. 2. 3. 4. 5. Pgas = Ph Pgas = Patm Pgas = Ph + Patm Pgas = Ph - Patm Pgas = Patm - Ph If 250 mL of NO is placed in a flask with O2, what volume of O2 is needed for complete reaction? 2 NO (g) 1. 2. 3. 4. 5. + O2 (g) 2 NO2 (g) 100 mL 125 mL 200 mL 250 mL Cannot be determined from the given information. If 250 mL of NO is placed in a flask with O2, what volume of O2 is needed for complete reaction? 2 NO (g) 1. 2. 3. 4. 5. + O2 (g) 2 NO2 (g) 100 mL 125 mL 200 mL 250 mL Cannot be determined from the given information. If an equal mass of each gas is put into a separate balloon, which will have the greatest volume? 1. 2. 3. 4. 5. Assume that they are all the same temperature and pressure. He H2 N2 Ne O2 If an equal mass of each gas is put into a separate balloon, which will have the greatest volume? 1. 2. 3. 4. 5. Assume that they are all the same temperature and pressure. He H2 N2 Ne O2 If equal masses of CH4, C2H6, and C3H8 are placed in a flask, which of the following is true? 1. 2. 3. 4. 5. PCH4 = PC2H6 = PC3H8 PCH4 ~ PC2H6 ~ PC3H8 PCH4 > PC2H6 > PC3H8 PCH4 < PC2H6< PC3H8 None of the above If equal masses of CH4, C2H6, and C3H8 are placed in a flask, which of the following is true? 1. 2. 3. 4. 5. PCH4 = PC2H6 = PC3H8 PCH4 ~ PC2H6 ~ PC3H8 PCH4 > PC2H6 > PC3H8 PCH4 < PC2H6< PC3H8 None of the above Arrange the gases according to increasing molecular speed. He (25°C) 1. 2. 3. 4. 5. He (100°C) Ne (25°C) He (25) < He (100) < Ne (25) < Ne (0) He (25) < He (100) < Ne (0) < Ne (25) Ne (0) < Ne (25) < He (25) < He (100) Ne (25) < Ne (0) < He (100) < He (25) Ne (0) < He (25) < Ne (25) < He (100) Ne (0°C) Arrange the gases according to increasing molecular speed. He (25°C) 1. 2. 3. 4. 5. He (100°C) Ne (25°C) He (25) < He (100) < Ne (25) < Ne (0) He (25) < He (100) < Ne (0) < Ne (25) Ne (0) < Ne (25) < He (25) < He (100) Ne (25) < Ne (0) < He (100) < He (25) Ne (0) < He (25) < Ne (25) < He (100) Ne (0°C) If a mixture of gas A and gas B is moved from flask 1 to flask 2, which of the following is true: 1. 2. 3. 4. 5. PA, PB, and Ptot decrease flask 1 PA, PB, and Ptot increase PA and PB decrease, Ptot remains the same PA, PB, and Ptot remain the same PA and PB remain the same, Ptot decreases flask 2 If a mixture of gas A and gas B is moved from flask 1 to flask 2, which of the following is true: 1. 2. 3. 4. 5. PA, PB, and Ptot decrease flask 1 PA, PB, and Ptot increase PA and PB decrease, Ptot remains the same PA, PB, and Ptot remain the same PA and PB remain the same, Ptot decreases flask 2 A gas initially at 2.0 atm is in an adjustable volume container of 10. L in volume. If the pressure is decreased to 0.50 atm, what is the new volume? 1. 2. 3. 4. 40. L 20. L 10. L 5.0 L Correct Answer: 1. 2. 3. 4. 40. L 20. L 10. L 5.0 L 1 V = constant P PV = constant Thus, 2.00 atm(10. L) = 0.50 atm (Vfinal) Vfinal = 2.00 atm(10. L)/0.50 atm = 40. L Assuming pressure is held constant, to what volume will a balloon initially at 1.0 L change if its temperature is decreased from 300 K to 75 K? 1. 2. 3. 4. 1.0 L 2.0 L 0.25 L 4.0 L Correct Answer: 1. 2. 3. 4. 1.0 L 2.0 L 0.25 L 4.0 L V = constant T V T = constant Thus, 1.0 L/300 K = (Vfinal)/75 K Vfinal = 75 K/(1.0 L)300 K = 0.25 L At standard temperature and pressure, how many moles of gas are present in a box with a volume of 112 L? 1. 2. 3. 4. 1.00 moles 2.00 moles 5.00 moles 0.200 moles Correct Answer: 1. 2. 3. 4. 1.00 moles 2.00 moles 5.00 moles 0.200 moles PV = nRT V = nRT P = (1mol)(0.08206 L·atm/mol·K)(273.15K) 1.000 atm Thus, at STP 22.41 L = 112 L 1.00 mol n n = 5.00 moles = 22.41L At standard temperature and pressure, a hot-air balloon is filled with helium only to a volume of 4480 L. How many grams of helium are needed to fill the balloon? 1. 2. 3. 4. 200. g 400. g 800. g 50.0 g Correct Answer: 1. 2. 3. 4. 200. g 400. g 800. g 50.0 g At STP 22.41 L = 4480 L 1.00 mol n n = 200. moles Since MW(He) is 4.0 g/mol Mass = (200. g)(4.0 g/mol) = 800. g A gas sample occupies a volume of 4.00 L at 20°C. The temperature at which the gas would double its volume is 1. 2. 3. 4. 10°C 40°C 288°C 313°C Correct Answer: 1. 2. 3. 4. 10°C 40°C 288°C 313°C V T = constant The temperature scale is absolute, however; Vinitial/ (Vfinal) = Tfinal/ (Tinitial) 2 = Tfinal/ 293 K Tfinal = 586 K, or 313°C N2(g) + 3 H2(g) 2 NH3(g) At STP, 16 L of N2 and 48 L of H2 are mixed. Assuming all the reactants are consumed, how many L of NH3 will be produced? 1. 2. 3. 4. 5. 8.0 L 16 L 24 L 32 L 64 L Correct Answer: 1. 2. 3. 4. 5. 8.0 L 16 L 24 L 32 L 64 L V n (constant P, T ) According to Avogadro’s law, mole ratios in the chemical equation will be volume ratios under identical conditions. Because the reactants are in a stoichiometric 3:1 volume ratio, the product will have stoichiometric equivalence. Thus, (16 L N )(2 mol NH /1 mol N ) = 32 L NH A container holds a mixture of oxygen, neon, and helium gases whose partial pressures are 150 torr, 300 torr, and 450 torr, respectively. The mole fraction of neon is 1. 2. 3. 4. 0.17 0.33 0.50 0.67 Pi = i Ptotal Correct Answer: 1. 2. 3. 4. 0.17 0.33 0.50 0.67 Pi = i Ptotal Xi = Pi/Ptotal Xi = (300 torr)/(150 + 300 + 450) torr Xi = 300 torr/900 torr = 0.33 A sample of He gas initially at STP is compressed to a smaller volume at constant temperature. What effect does this have on the rms speed of the atoms? 1. Increases 2. Decreases 3. No effect Correct Answer: 1. Increases 2. Decreases 3. No effect The rms speed is directly proportional to the square root of the temperature, which does not change in this example. u= 3 RT M An unknown gas effuses at half the rate of helium. This gas is likely to be which of the following? 1. 2. 3. 4. 5. H2 CH4 Ne O2 Ar Correct Answer: 1. 2. 3. 4. 5. H2 CH4 Ne O2 Ar r1 r2 = M2 M1 (r1/2)2 =M2/M1 M2= (r1/r2)2M1 M2= (2/1)2(4.0 g/mol) = 16.0 g/mol Therefore it could be CH4 Real gases deviate from ideal behavior at __________ and _________. 1. High temperature; low pressure 2. Low temperature; high pressure 3. High temperature; high pressure 4. Low temperature; low pressure Correct Answer: 1. High temperature; low pressure 2. Low temperature; high pressure 3. High temperature; high pressure 4. Low temperature; pressure and At lowlow temperature high pressure, intermolecular forces increase as the molecules get closer together.