Measuring Heat
advertisement

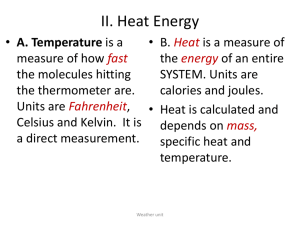
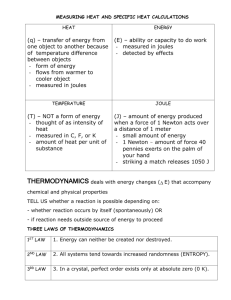
Name_________________________________ Chemistry Date______________________ Unit: The Chemistry of Biofuels Measuring Heat This is a key- delete everything in red if you’d like to use this in your class. These are guided notes for the “content day” of the biofuels PBL unit. Options for facilitation include but are not limited to: overhead with transparency (traditional) or discovery or text driven (students seeking info themselves). Unit for measuring heat and all other forms of energy is the ___joule, J_____________. Another unit for measuring heat is the_________calorie, cal________________. To convert between these two units: 1 cal = 4.184 J 1000cal = 4184 J = 1 dietary Calorie= 4.2 kJ Specific Heat (cp) -the amount of energy required to ____raise/increase___________the temperature of one gram of a substance by one Celsius degree (1°C) or one kelvin (1 K).. *The amount of energy transferred as heat during a temperature change depends on the nature of the material changing temperature, and on its mass. Values are typically given in joules per gram per Celsius degree, J/(g•°C), or joules per gram per kelvin, J/(g•K). You can also use calories in place of joules. We can calculate specific heat using the formula: cp = __q___ m∆T q= heat in Joules (J) m= mass in grams (g) ∆T= change in temperature= final T – initial T ∆T is ALWAYS positive for endo (adding heat to system) and negative for exo (subtracting heat from system) *if heat energy is given in kJ AND mass is given in kg, you don’t have to convert. ie. _kJ__ is the same as J__ because the units cancel. kg ·˚C g · ˚C 1 Name_________________________________ Chemistry Date______________________ Unit: The Chemistry of Biofuels Specific Heat Example Problems 1. A 4.0 g sample of glass was heated from 274 K to 314 K, a temperature increase of 40. K, and was found to have absorbed 32 J of energy as heat. What is the specific heat of this type of glass? cp = __q___ = 32 J_____ = 0.2 J/g·K m∆T (4.0g)(40K) 2. The specific heat of toluene, C7H8, is 1.13 J/g·˚C. How many joules of heat are needed to raise the temperature of 40.0 grams of toluene from 10.4˚C to 28.0˚C? Rearranging the equation we get q= cp m∆T = (1.13 J/g·˚C)(40.0g)(28.0˚C -10.4˚C) = 795.52 J 3. A total of 15.7kJ (15700J) of heat are added to a 137.6 g sample of silver at 23˚C. What is the final temperature of the silver? The specific heat of silver is 0.24 J/g·˚C. Rearranging the equation (and breaking ∆T into final and initial temperature (Tf and Ti), we get Tf = __q___ + Ti = ______15700 J_____ + 23˚C = 498˚C cp m (0.24 J/g·˚C) (137.6 g) Quick check: How would we convert to K? Answer: Add 273˚C, 771 K 2