The pH Scale
advertisement

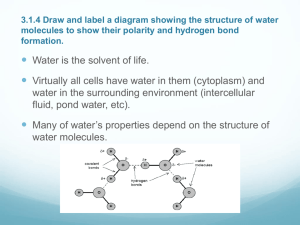
Biochemistry Water and the Fitness of the Environment Water: The molecule that supports life on this planet! • Water is the biological medium for all life on Earth • All living organisms require water more than any other substance 2 FOUR Emergent Properties of water contribute to Earth’s suitability for life 1. Water’s cohesive & adhesive behavior 2. Water’s ability to moderate temperature 3. Water’s expansion upon freezing 4. Water’s versatility as a solvent. 3 What causes water molecules to both cohere and adhere? • Yep! Our good buddy the intermolecular force (IMF) named hydrogen bonding • Cohesion is when water molecules stick to each other. • Adhesion is when water molecules stick to some other type of substance like plant cell walls. Animation by scrolling over image 4 What causes water molecules to both cohere and adhere? • Surface tension is a measure of how hard it is to break the surface of a liquid. • Surface tension is a consequence of cohesion. What causes it? Go ahead, guess! Hydrogen bonding, yet again! 5 How does water moderate temperature? • Water has a high heat capacity. • Heat capacity is the amount of heat required to raise 1 gram of water by 1o Celsius (1 calorie = 4.184 J) • What does that mean? It means water can absorb large quantities of heat without much change in its own temperature, thus it’s a good thermo regulator. • But, why? Guess again! 6 It’s a 2-way street! • Water’s high specific heat can be traced to hydrogen bonding (IMFs) – Heat is absorbed when hydrogen bonds break – Heat is released when hydrogen bonds form • The high specific heat of water minimizes temperature fluctuations to within limits that permit life 7 Ever sweat? Ever perspire? • Evaporation is transformation of a substance from liquid to vapor • Heat of vaporization is the heat a liquid must absorb for 1 g to be converted to vapor • As a liquid evaporates, its remaining surface cools, a process called evaporative cooling • Evaporative cooling of water helps stabilize temperatures in organisms (sweating, panting, etc.) and bodies of water 8 Water is a freak! It expands upon freezing! • Ice floats in liquid water because hydrogen bonds in ice are more “ordered” forming a hexagonal shape with a hole in the middle, making ice less dense. • Water reaches its greatest density at 4°C, which is excellent news if you’re a fish! • If ice sank, all bodies of water would eventually freeze solid, making life impossible on Earth Water is a freak! It expands upon freezing! Hydrogen bond Ice: Hydrogen bonds are stable Liquid water: Hydrogen bonds break and re-form Water’s Versatility as a Solvent • A solution is a liquid that is a homogeneous mixture of substances • A solvent is the dissolving agent of a solution or “dissolver” • The solute is the substance that is dissolved or “disolvee” • An aqueous solution is one in which water is the solvent 11 Water’s Versatility as a Solvent • Water is a versatile solvent due to its polarity, which allows it to form hydrogen bonds easily. • When an ionic compound is dissolved in water, each ion is surrounded by a sphere of water molecules called a hydration shell 12 Water’s Versatility as a Solvent • Water can also dissolve compounds made of nonionic polar molecules • Even large polar molecules such as proteins can dissolve in water if they have ionic and polar regions 13 Hydrophilic vs. Hydrophobic • A hydrophilic substance is one that has an affinity for water • A hydrophobic substance is one that does not have an affinity for water; “fears water” • Oil molecules are hydrophobic because they have relatively nonpolar bonds • A colloid is a stable suspension of fine particles in a liquid (like milk—fat suspended in water—Yummy!) 14 Acids, Bases and Buffers. Oh, my! ACIDIC AND BASIC CONDITIONS AFFECT LIVING ORGANISMS • A bonded hydrogen atom within a water molecule can shift between two water molecules (from one molecule to the other). 15 Acids, Bases and Buffers. Oh, my! – The hydrogen atom leaves its electron behind and is transferred as a proton, or hydrogen ion (H+) – The molecule with the extra proton is now a hydronium ion (H3O+), though it is often represented as H+ – The molecule that lost the proton is now a hydroxide ion (OH–) 16 Acids, Bases and Buffers. Oh, my! • Though statistically rare, the dissociation of water molecules has a great effect on organisms • About 2 in every billion water molecules exist as H+ and OH– • Changes in concentrations of H+ and OH– can drastically affect the chemistry of a cell 17 Acids, Bases and Buffers. Oh, my! • Concentrations of H+ and OH– are equal in pure water • Adding certain solutes, called acids and bases, modifies the concentrations of H+ and OH– • Biologists use something called the pH scale to describe whether a solution is acidic or basic (the opposite of acidic; also known as alkaline) 18 Acids, Bases and Buffers. Oh, my! Simply put, • An acid is any substance that increases the H+ concentration of a solution • A base is any substance that reduces the H+ concentration of a solution Bleach 19 Acids, Bases and Buffers. Oh, my! Buffers • The internal pH of most living cells must remain close to pH 7 • Buffers are substances that RESIST changes in concentrations of H+ and OH– in a solution, therefore they RESIST a change in pH • Most buffers consist of an acid-base pair that reversibly combines with H+ 20 The pH Scale • An acid is any substance that increases the H+ concentration of a solution • A base is any substance that reduces the H+ concentration of a solution • The scale was designed to compare WEAK acids and bases. 21 The pH Scale • In any aqueous solution at 25°C the product of H+ and OH– is constant and can be written as the autoionization constant of water [H+][OH–] = 10–14 • The pH of a solution is defined by the negative logarithm of H+ concentration, written as pH = –log [H+] • For a neutral aqueous solution, [H+] is 10–7, so pH = –(–7) = 7 22 The pH Scale • Acidic solutions have pH values less than 7 • Basic solutions have pH values greater than 7 • Most biological fluids have pH values in the range of 6 to 8 23 The pH Scale • Each increase (toward more acidic or more basic – away from 7 in either direction) on the pH scale increases by a factor of 10. So an acid with a pH of 2 is 100 times more acidic than an acid with a pH of 4. 24 Created by: René McCormick National Math & Science Dallas, TX