Explain 1

The Bohr Model meets the Quantum Mechanical Model
The Quantum Mechanical
Model adds details to the
Bohr Model.
It identifies ____________, which are s, p, d, and f, on each energy level.
Sublevels are composed of
___________________,
, which can hold ____ electrons.
vs.
Why does a hamburger have lower energy than a steak?
Because it’s in the ground state!
The farther away an electron is from the nucleus, the higher the energy it has. An electron in its normal energy level is in its ground state.
Rules governing ground state locations of electrons and “Do” and “Don’t”:
Pauli Exclusion Principle: each
Hund’s Rule: you must place one Aufbau Principle: electrons fill the orbital can hold TWO electrons with opposite spins.
Do this electron in each orbital before pairing them. This rule is often called the
“Empty Bus Seat Rule.” lowest energy orbitals first. This rule is often called the “Lazy Tenant Rule.”
Do this
Do this
Not this Not this Not this
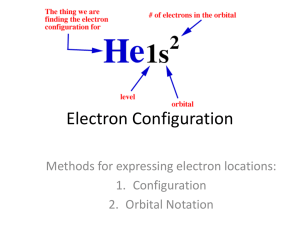
Expressing Electron Configuration in Different Notations:
These are two of the ways to write out an electron configuration for an atom. The Orbital Diagram Notation shows all energy levels, sublevels, and orbitals, and how the electrons are arranged in them. Suppose you have oxygen with 8 electrons. The following shows how each orbital is filled with the appropriate number of electrons:
Orbital Diagram Notation for Oxygen
1 s 2 s 2 p
Standard or Shorthand Electron Configuration Notation writes out the energy levels and sublevels of the electrons, stating the number of electrons in each location as a superscript in order of increasing energy.
Suppose you have oxygen with 8 electrons. The following shows the standard/shorthand electron configuration notation:
Shorthand or Standard Electron Configuration Notation for Oxygen
1 s 2 2 s 2 2 p 4
Example.
Atomic Number: __ 11 __ Element: ___ Sodium ______________ Symbol: ___ Na _____
Orbital Diagram of Electron Configuration:
↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑
1s 2s 2p 3s
Shorthand Electron Configuration Notation:
1s
2
2s
2
2p
6
3s
1
Whole Class Practice.
Atomic Number: __28__ Element: ________ ______________ Symbol: _________
Orbital Diagram of Electron Configuration: Shorthand Electron Configuration Notation:
1s 2s 2p
Electron Configuration Practice
Please write the correct standard electron configuration and noble gas configuration for each of the following elements.
Element Orbital Diagram Electron Configuration Standard/shorthand Electron
Configuration
1.
Aluminum
2.
3.
Argon
Carbon
4.
Magnesium
5.
6.
Nickel
Bromine