Document
advertisement

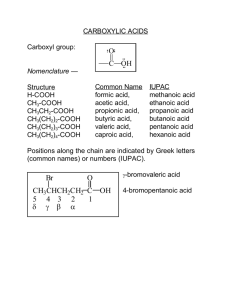
LECTURE 2 THEME: Structure and chemical properties of carboxylic acids. Heterofunctional compounds. Lecturer: Yevheniya. B. Dmukhalska 1. 2. 3. 4. 5. 6. 7. 8. Plan Nomenclature of carboxylic acids Physical properties of carboxylic acids. Classification of carboxylic acids Methods of preparation of carboxylic acids Chemical properties of carboxylic acids. Heterofunctional compounds. Hydroxy-acids, nomenclature, isomerism, chemical properties and specific reactions for hydroxy-acids. Introduction of optical isomerous. Mirror (optical) isomerism. Asymmetric carbon atom. Properties of enantiomers. Carboxylic acids Carboxylic acids are compounds whose characteristic functional group is the carboxyl group - COOH , example: Common formula of carboxylic acid: Nomenclature of carboxylic acids Nowhere in organic chemistry are common names used more often than with the carboxylic acids. Systematic names for carboxylic acids are derived by counting the number of carbons in the longest continuous chain that includes the carboxyl group and replacing the -e ending of the corresponding alkane by -oic acid. Table 1. Systematic and common names of some carboxylic acids Classification of carboxylic acids : 1. From the nature of hydrocarbon radical: a) saturated acid is acid, which has only simple bonds in molecule. Example: formic acid, buthanic acid; b) unsaturated acid is an acid, which has both as simple bonds and duble bonds in molecule. Example: oleic acid, linoleic acid, linolenic acid, arashdonic acid; c) aromacic acid is acid, which contain aromatic ring. Example: benzoic acid. 2. The number of carboxyl groups a) monocarboxylic acid is acid, which has one carboxylic group in molecule. Example: acetic acid, formic acid, buthanic acid; b) dicarboxylic acid is acid, which has two carboxylic group in molecule. Example: oxalic acid, malonic acid. The names of some saturated monocarboxylic acids Structural formula Name of nomenclature O H C trivial substitute rational formic acid methanoic acid - acetic acid etanoic acid acetic acid propionic acid propanoic acid methylacetic acid oil acid butanoic acid ethylacetic acid iso oil acid 2-methylpropanoic acid dimethylacetic acid valeric acid pentanoic acid propylacetic acid iso valeric acid 3-methylbutanoic acid methylethylacetic acid OH O H3C C OH O H3C CH2 C OH O H3C CH2 3 CH2 C 2 1 C H3C CH OH O OH CH3 O H3C CH2 4 3 H3C CH 2 CH2 CH2 1 C CH3 CH2 O OH C OH CH3-(CH2)4-COOH capronic acid hexanoic acid CH3-(CH2)10-COOH lauric acid dodecanoic acid CH3-(CH2)12-COOH myristic acid tetradecanoic acid CH3-(CH2)14-COOH palmitic acid hexadecanoic acid CH3-(CH2)16-COOH stearic acid octadecanoic acid n-butylacetic acid The names of some unsaturated monocarboxylic acids Name of nomenclature Structural formula trivial CH2=CH-COOH CH2 C COOH substitute acrylic acid propenoic acid methacrylic acid 2-methylpropenoic acid vinyl acetic acid 3-butenoic acid crotonic acid trans-2-butenoic acid iso crotonic acid cus-2-butenoic acid propiolic acid propionoic acid tetrolic acid 2-butynoic acid oleic acid cus-9-octadecenoic acid Linoleic acid cus-9-cus-12octadecadienoic acid linolenic acid cus-9-cus-15octadecatrienoic acid CH 3 CH2=CH-CH2-COOH H COOH C C H CH3 H H C CH3 CH C 4 3 2 CH3 C C COOH COOH CH3 CH (CH2)7 COOH CH CH2 CH (CH2)7 COOH 1 CH (CH2)7 CH3 (CH2)4 C CH CH COOH CH CH2 CH CH2 CH3 CH CH CH2 CH CH (CH2)7 COOH The names of some dicarboxylic acids Structural formula Name of nomenclature trivial substitute HOOC-COOH oxalic acid ethandioic acid HOOC-CH2-COOH malonic acid propandioic acid HOOC-CH2-CH2-COOH succinic acid butandioic acid HOOC-CH2-CH2-CH2-COOH glutaric acid pentandioic acid HOOC-CH2-CH2-CH2-CH2-COOH adypinic acid hexandioic acid HOOC-(CH2)5-COOH pimelic acid heptadioic acid HOOC-(CH2)6-COOH cork acid octandioic acid maleic acid cus-butendioic acid fumaric acid trans-butendioic acid phthalic acid 1,2-benzoldicarbonic acid iso phthalic acid 1,3-benzoldicarbonic acid H H C C COOH HOOC H COOH C HOOC C H COOH COOH COOH COOH Physical properties of carboxylic acids. The melting points and boiling points of carboxylic acids are higher than those of hydrocarbons and oxygen-containing organic compounds of comparable size and shape and indicate strong intermolecular attractive forces. The hydroxyl group of one carboxylic acid molecule acts as a proton donor toward the carbonyl oxygen of a second. In a reciprocal fashion, the hydroxyl proton of the second carboxyl function interacts with the carbonyl oxygen of the first. Methods of preparation of carboxylic acids. 1. Oxidation of alkylbenzenes. 2. Oxidation of primary alcohols. Potassium permanganate, potassium chromate and chromic acid convert primary alcohols to carboxylic acids by way of the corresponding aldehyde. 3. Oxidation of aldehydes. Aldehydes are particularly sensitive to oxidation and are converted to carboxylic acids by a number of oxidizing agents, including potassium permanganate and chromic acid. 4. Synthesis of carboxylic acids by the preparation and hydrolysis of nitriles. Once the cyano group has been introduced, the nitrile is subjected to hydrolysis. Usually this is carried out in aqueous acid at reflux. Chemical properties of carboxylic acids. Formation of acyl chlorides. Thionyl chloride reacts with carboxylic acids to yield acyl chlorides. Formation of acyl chlorides. Reaction with halo-compounds: Reduction reaction. Carboxylic acids are reduced to primary alcohols by the powerful reducing agent lithium aluminum hydride. Acidity: Iontzation: Reactions involving the ОН-bond a) b) Reactions involving the ОН-bond Important reaction of carboxylic acids involving the ОН bond - the reaction with bases to give salts. Another important reaction involving this bond is the reaction of carboxylic acids with diazomethane. The products of this reaction are the methyl ester and nitrogen. ESTERIFICATION This page looks at esterification - mainly the reaction between alcohols and carboxylic acids to make esters. α-halogenation of carboxylic acids The enol content of a carboxylic acid is far less than that of an aldehyde or ketone, and introduction of a halogen substituent at the -carbon atom requires a different set of reaction conditions. Bromination is the reaction that is normally carried out, and the usual procedure involves treatment of the carboxylic acid with bromine in the presence of a small amount of phosphorus trichloride as a catalyst. This method of α bromination of carboxylic acids is called the Hell–Volhard– Zelinsky reaction. Decarboxylation of carboxylic acids. The loss of a molecule of carbon dioxide from a carboxylic acid is known as decarboxylation. The formation amides. The most common reaction of this type is the reaction of carboxylic acids with ammonia or amines to give amides. When ammonia is bubbled through butyric acid at 1850, butyramide is obtained in 85% yield. The reaction involves two stages. At room temperature, or even below, butyric acid reacts with the weak base ammonia to give the salt ammonium butyrate. This salt is perfectly stable at normal temperatures. However, pyrolysis of the salt results in the elimination of water and formation of the amide. O O C H2C H2C OH OH C O succinic acid + NH3 t N H O sukcynimide + H2O Reaction formation carboxylic acid anhydrides. Acid anhydrides are the most reactive carboxylic acid derivatives. NaOH + C6H5COONa H2O O C2H5OH; H+ C6H5 + C H2O OC2H5 ethylbenzoath O PCl5 C6H5 + C HCl + POCl3 Cl benzoilchloride C6H5COOH benzoic acid O C6H5 C (CH3CO)2O; H+ O C6H5 + 2 CH3 C O anhydride of benzoic acid COOH H2 HOOC CH2 CH2 COOH succinic acid HOOC CH CH COOH Br2 HOH; H+ CH HOOC CH CH2 malic acid COOH CH Br Br 2, 3 -dibromsuccinic acid HCl HOOC CH CH2 COOH chlorsuccinic acid COOH COOH butendioic acid [O] HOOC KMnO4 CH CH COOH racemic acid 8. Carboxylic acid derivatives. These classes of compounds are classified as carboxylic acid derivatives. All may be converted to carboxylic acids by hydrolysis. Functional Group is any part of an organic compound, which is not а carbon-hydrogen or carbon-carbon single bon. There are mono-, poly- and heterofunctional group in the structure of organic compounds: Monofunctional group – contains only 1 functional group. C2H5—OH Polyfunctional group – contains several similar functional group. H2C CH H2C OH OH OH Heterofunctional group – contains several different functional group. Sphingosine Biological role: Heterofunctional compounds are widespread in the nature. They are in fruits and vegetable leafs. Also they are formed in body. So, the lactic acid is product of transformation glucose (glycolysis) in human body. A malic and citric acid formed in a cycle of tricarboxylic acids, which is also known as citric acid cycle or Krebs' cycle. Hydroxo acids such as: pyruvic acid, acetoacetic acid, oxaloacetic acid, -ketoglutaric acid are important in metabolism of carbohydrates. Hydroxyacids Hydroxyacids are the derivatives of carboxyl acids that contain –OH group (1 or more). β 3 H3C α 2 1 CH C OH O OH 2-hydroxypropanoic acid α-hydroxypropanoic acid glycolic acid, hydroxyacetic acid, hydroxyethanoic acid lactic acid, α- hydroxypropanoic acid, 2- hydroxypropanoic acid tartaric acid α,α’-dihydroxysuccinic acid, 2,3-dihydroxybutandioic acid, citric acid, 2-hydroxy-1,2,3-propantricarboxylic acid malic acid, hydroxysuccinic acid hydroxybutanedioic acid In a row of hydroxyacids often found the optical isomery. D-tartaric acid L-tartaric acid mezo-tartaric acid Methods of preparation of hydroxyacids: 1. Hydrolysis of α-halogenoacids O H3C C CH O + NaOH H2O H3C CH OH Cl C + NaCl OH OH lactic acid 2. Oxidations of diols and hydroxyaldehydes H3C 3. CH CH2 OH OH [O] O H3C CH [O] C O H3C H OH CH OH C OH Hydration of α,β-unsaturated carboxylic acids O CH2 CH C OH O + + H2O H H2C OH CH2 C OH β-hydroxypropanoic acid 4. Hydrolysis of hydroxynitriles (cyanohydrins) Physical and chemical properties of hydroxycarboxylic acid For physical properties of hydroxycarboxylic acids are colorless liquids or crystalline substance, soluble in water. Chemical properties: in the molecule of hydroxyacids ether – OH group or carboxyl group can react. Carboxyl group can react forming: a) salts: O H2C CH2 C + NaOH OH O H2C CH2 C ONa OH + H2O OH sodium β-hydroxypropanoic acid O 2 H2C CH2 C + 2 Na OH OH O 2 H2C CH2 C ONa OH + H2 OH O H2C CH2 C O O 2 H2C CH2 C Mg + H2O + MgO O OH OH H2C CH2 C O OH O H2C O CH2 C OH + NaHCO3 H2C CH2 C ONa OH + H2CO3 OH H2O CO2 b) Ester formation: O H2C CH2 C + HO OH OH CH3 O H2C CH2 C O OH CH3 + H2O Methyl-β-hydroxypropanoate c) Amides formation: O H2C CH2 C OH + NH3 t=200o O H2C OH CH2 C NH2 OH + H2O amide of β-hydroxypropanoic acid II. –OH group reaction: a) hydrohalogens (HCl, HBr, HI, HF) O H2C CH2 C + HCl O H2C OH OH CH2 C OH + H2O Cl b) can oxidize O H2C OH CH2 [O] C O HC OH O CH2 C + H2O OH β-oxopropanoic acid Related to heat of: 1. α-hydroxyacids lactic acid 2. β-hydroxyacids 3. γ-hydroxyacids lactide Decomposition α-hydroxyacids O H O СH3 С C H O к.H2SO4 O CH3 C t + H OH OH Ethanal HCOOH к. Н2SO4, t Ñ ÑH2 COOH ê. H2SO4 t formic acid CO + H 2O OH HOOCH2 C H C O O H C OH + HOOCH2 C C CH2 COOH acetidicarbonic acid COOH CO H2O 2 CO2 H3 C C CH3 O Representatives of hydroxyacids: lactic acid. lactic acid is a trivial name CH C because at first it was extracted from milk. It OH is present in yogurt, sour milk and other milk OH products. It can form in muscles during hard and prolonged work. Salts of milk acid are used in medicine. O Malic acid. It is present in green apples and C CH CH2 C OHsome berries. It takes part in biological OH processes in human organisms and organisms of other alive creatures. It is used in medicine for synthesis of some medical preparations. O Tartaric acid . It is present in grape. It is C used in medicine for synthesis of some OH CH OH medical preparations. O H3C O HO CH OH O C OH O C OH CH2 HO C O C CH2 O C OH OH Citric acid . It is present in orange, lemon and other citric fruits. It takes part in biological processes in human organism. Phenolacids. Phenolacids are the derivatives of aromatic carboxyl acids that contain –OH group (1 or more). o-hydroxycinnamic acid salicylic acid, 2-hydroxybenzoic acid 4-hydroxybenzoic acid 3,4,5-trihydroxybenzoic acid, gallic acid Chemical properties of phenolacids: Chemical properties of phenolacids due to the presence in their structure of carboxyl group, phenolic hydroxyl and the aromatic nucleus. COOH COONa + NaHCO3 OH + CO2 + H2O OH salicylic acid COOH + FeCl3 OH COO O.. H OH salicylic acid 3 complex helatic salt of salicylic acid (violet colour) Decarboxylation COOH Fe + 3HCl t + OH phenol CO2 The best known aryl ester is O-acetylsalicylic acid, better known as aspirin. It is prepared by acetylation of the phenolic hydroxyl group of salicylic acid: Aspirin possesses a number of properties that make it an often-recommended drug. It is an analgesic, effective in relieving headache pain. It is also an antiinflammatory agent, providing some relief from the swelling associated with arthritis and minor injuries. Aspirin is an antipyretic compound; that is, it reduces fever. Each year, more than 40 million lb of aspirin is produced in the United States, a rate equal to 300 tablets per year for every man, woman, and child. Oxoacids To oxoacids include aldehydo- and ketonoacids. These compounds include in the structure of the carboxyl group, aldehyde functional group or ketone functional group. glyoxylic acid, oxoethanoic acid acetoacetic acid, 3-oxobutanoic acid, β-ketobutyric acid oxalacetic acid, oxobutanedioic acid, ketosuccinic acid pyroracemic acid, 2-oxopropanoic acid γ-ketovaleric acid, 4-oxopentanoic acid, levulinic acid Chemical properties of oxoacids 1. Decarboxylation of α-oxoacids O CH3 C COOH O conc. H2SO4, t CH3 H acetaldehyd pyroracemic acid 2. C + CO2 Decarboxylation of β-oxoacids O CH3 C CH2 COOH acetoacetic acid t - CO2 CH3 C O acetone CH3 Stereochemistry The three-dimensional shape of an organic molecule can have а dramatic effect upon its reactivity. In fact, the study of the shapes of organic molecules is so important that it forms а separate subdiscipline within organic chemistry — stereochemistry, from the Greek word “” (stereos), meaning solid; this chapter will be devoted to the study of organic molecules in three dimensions. Stereoisomers Compounds which differ in the threedimensional arrangement of the atoms in space but have the same connectivity are termed stereoisomers. Stereoisomers are compounds that have the same sequence of covalent bonds and differ in the relative disposition of their atoms in space. 1. 2. There are two major causes of stereoisomerism: the presence of "structural rigidity" in а molecule. Structural rigidity is caused by restricted rotation about chemical bonds. It is the basis for cis - trans stereoisomerism, а phenomenon found in some substituted cycloalkanes and some alkenes; the presence of а chiral center in а molecule. COFORMATION The methyl groups can rotate freely about the central C–C bond. Structures that differ only by rotation about one or more single bonds are defined as conformations of a compound. For example: Ethane has two conformations: eclipsed structure, which is more higher in energy than the more stable staggered structure. Configuration The stereoisomers that are not easily interconverted are called configurational isomers. Mirror Images The concept of mirror images is the key to understanding molecular handedness. All objects, including all molecules, have mirror images. The mirror image of an object is the object’ reflection in а mirror. For example: human hands. Chirality The general property of "handedness" is called chirality. An object that is not superimposable upon its mirror image is chiral. If an object and its mirror image can be made to coincide in space, then they are said to be achiral. A person’s left and right hands are not superinposable upon each other. Any organic molecule containing а single carbon atom with four different groups attached to it exhibits chirality. А chiral center is an atom in а molecule that has four different groups tetrahedrally bonded to it. It is asymmetric atom. Enantiomers are stereoisomers whose molecules are nonsuperimposable mirror images of each other. Properties of enantiomers Enantiomers are said to be optically active because of the way they interact with plane-polarized light. An optically active compound is а compound that rotates the plane of polarized light. Ordinary light waves - that is, unpolarized light waves - vibrate in all planes at right angles to their direction of travel. Plane-polarized light waves, by contrast, vibrate in only one plane at right angles to their direction of travel. Polarimeter An enantiomer that rotates plane-polarized light to the right is said to be dextrorotatory (the Latin dexter means "right"). An enantiomer that rotates plane-polarized light to the left is said to be levorotatory (the Latin laevus means "left"). А plus or minus sign inside parentheses is used to denote the direction of rotation of planepolarized light by а chiral compound. The notation (+) means rotation to the right (clockwise), and (-) means rotation to the left (counterclockwise). Thus the dextrorotstory enantiomer of glucose is (+)-glucose. An equimolar mixture of two enantiomers is called а racemic mixture, or а racemate. Since а racemic mixture contains equal numbers of dextrorotating and levorotating molecules, the net optical rotation is zero. А racemic mixture is often specified by prefixing the name of the compound with the symbol ( ); Diastereomers Diastereomers - stereoisomers that are not mirror images of each other. Epimers are diastereomers that differ only in the configuration at one chiral center. In general, а compound that has n chiral centers may exist in а maximum of 2n stereoisomeric forms. For example, when three chiral centers are present, at most eight stereoisomers (23 = 8) are possible (four pairs of enantiomers).