Energy & Heat Jeopardy Game
advertisement

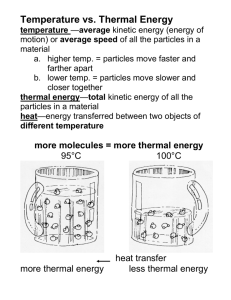
Vocabulary Temperature Energy Transfer Particles in Matter Thermal Energy 10 10 10 10 10 20 20 20 20 20 30 30 30 30 30 40 40 40 40 40 50 50 50 50 50 What is solar energy? energy given off by the sun What is thermal energy? the energy of the motion of particles in matter What is heat? bundles of energy that move through matter and space Explain radiation and infrared radiation? radiation is the transfer of thermal energy from one piece of matter to another; infrared radiation are the bundles of energy that transfer heat What are convection and conduction, and how are they different? Conduction is the transfer of thermal energy by particles of matter bumping into each other. Convection is the energy transfer by hot particles moving in a liquid or gas. They are different because matter has to be touching for conduction to take place. What is temperature? a measure of the average energy of motion of matter How is the average energy of motion of matter measured? with a thermometer What is an insulator? How can clothes be insulators? It is a material that keeps thermal energy from being transferred. Clothes keep the heat from escaping your body on cold days. Tell me about the temperatures in a house with a metal roof verses a shingle roof? Talk about summer and winter. Metal is a good conductor of thermal energy, so a metal roof would create a greater temperature in the summer and a lesser temperature in the winter. Shingles are a better insulator, so the transfer of energy wouldn’t be as great as metal. These temperatures were taken at one-hour intervals at a house with a metal roof. What times could you infer that these A good inference wouldwere be that the first measurements taken? temperature reading was at 11:00 am, then 1:00pm, then 3:00pm, then 5:00pm, then 7:00pm, then 9:00pm, then 11:00pm. Think about things you do throughout the day. Name three of your actions that take a lot of energy and three that only take a little energy? examples of a lot: jumping, running, playing examples of a little: thinking, writing, sleeping What energy transfer process allows for you to have the perfect temperature bath- not too cold? convection because energy is transferred by the hot particles moving in a liquid. Which picture shows heat being transferred through infrared radiation? Why? The sun transfers heat through infrared radiation. Infrared radiation are the bundles of energy that transfer heat. The faucet transfers through convection. People use solar energy for inexpensive heating. What type of energy transfer is happening in these pictures? infrared radiation Describe in detail all the energy transfers that are taking place in this picture. Conduction between the hand and handle of the pot. Since the pot is not touching the burner, there is no conduction between the burner and pot. Convection is happening in the water and as the warm air rises to the ceiling. Radiation is happening as heat is transferred from the burner. When do particles in matter move faster? when heat is added Put the pictures in order from the slowest moving particles to the fastest moving particles. the ice particles are moving the slowest and have the least thermal energy, then the juice, and the boiling water has the most thermal energy. Therefore the boiling water has the fastest moving particles. What’s happening to the particles in the air between 9:00am and noon? Since the temperature is rising, the particles in the air are moving faster! Remember, volume is the amount of space matter takes up. If you have the same number of molecules of water, does it always have the same volume? Explain. No. If the water is a solid, like ice, the particles will stick closer together and take up less space. When it is liquid water, the particles spread out a little and take up a little more space. When water is a gas, like water vapor, the particles spread way out and take up much more space. How are thermal energy and temperature related? Thermal energy is the energy of the motion of particles in matter. Temperature is a measure of the average energy of motion of matter. The higher the thermal energy, the higher the temperature. If you wanted to know about how much thermal energy some matter had, what could you do? use a thermometer and take the temperature of the matter Tell me what you can infer about the particles of water based on the thermometer readings? The second glass has the lowest temperature, therefore, it has the lowest average energy of motion. The last glass has the highest temperature, therefore, it has the highest average energy of motion. The particles in the last glass are moving the fastest. How can you tell which picture has air with the highest thermal energy? You could measure the temperature of the air in each place. The place with the highest temperature has the highest amount of thermal energy. The average energy of motion in water is increasing with each picture. We know their thermal energies are different because ice, water, and water vapor have different average temperatures. What can we infer has happened to the water in each picture to end up with different thermal energies? You can infer that heat was added and the particles of the liquid are moving faster than the ice. And the particles of the water vapor are moving faster than the water. What is expansion and contraction? Which goes with more thermal energy, and which goes with less thermal energy? Expansion is when particles spread out and take up more space. When matter has more thermal energy, it expands. Contraction is when particles scrunch up and take up less space. When matter has less thermal energy, it contracts.