Acetyl CoA
advertisement

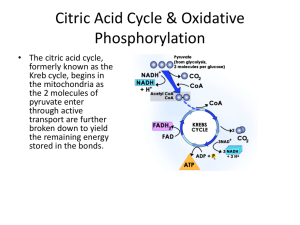
Enzymes Number Size >104 Coenzymes 15-20 Macromolecules (104 Small molecules (102 to104) to106) Proteins Structure (ribozymes) Most are heterocyclic organic compounds Building Blocks Amino acids (nucleic acids) Vitamins, modified amino acids, nucleotides, metals Active Center Amino acid side chains, metal cofactors Special chemical groups Always restored to Chemically changed by Recycling original form during a enzyme action; Prosthetic single cycle groups 1 Acetyl CoA O CH3C-SCoA O CH3C-X + H-SCoA Able to recognize CoASH Coenzyme A 2 CoA Acetyl CoA O 1. An important molecule in metabolism CH3C~SCoA 2. Its main use is to convey the C atoms within the acetyl group to the TCA cycle to be oxidized for energy production. 3. In chemical structure, acetyl-CoA is the thioester between coenzyme A (a thiol) and acetic acid (an acyl group carrier). 4. Acteyl-CoA is produced during the second step of aerobic cellular respiration, pyruvate decarboxylation, which occurs in the matrix of the mitochondria. Pyruvate dehydrogenase Pyruvate +NAD+ + CoA---------Acetyl-CoA +CO2 +NADH NAD+ is a common oxidant 5. C~S is a high energy bond energetically favorable for group transfer. 6. It has to couple ATP hydrolysis to regenerate. 3 Nicotinamide Adenine Dinucleotide Coenzymes (NAD; NADP NADH;NADPH) NADH Able to recognize 4 NAD / NADP 1. NAD is a dinucleotide, consists of two nucleotides joined through their phosphate groups. 2. In metabolism, NAD+ is involved in redox reactions, carrying electrons from one reaction to another. 3. NAD+ is an oxidizing agent – it accepts electrons from other molecules and becomes reduced, to form NADH. 4. NADH is a reducing agent – it can donate electrons. 5. Electron transfer reactions are the main function of NAD+. 6. NADPH is NADH with an extra phosphate group on the 2’ site of the ribose ring that carries the adenine moiety. 7. NADPH usually provides the reducing power for biosynthetic reactions. 5 FAD and FMN 6 Able to recognize FAD / FMN 1. FAD is a redox cofactor involved in several important reactions in metabolism. 2. FAD can exist in two different redox states and its biochemical role usually involves changing between these two states. FAD can be reduced to the FADH2, whereby it accepts two H atoms. 3. FMN functions as prosthetic group of various oxidoreductases such as NADH dehydrogenase. 4. During catalytic cycle, the reversible interconversion of oxidized (FMN), semiquinone (FMNH•) and reduced (FMNH2) forms occurs. 5. FMN is a stronger oxidizing agent than NAD+ and is particularly useful because it can take 7 part in both one and two electron transfers. Metabolism 8 Glycolysis 9 Glycosis 10 GAP dehydrogenase Aldehyde Strong oxidant carboxylic acid 11 ATP production 1,3-BPG is an energy –rich molecule with a greater phosphoryl-transfer potential than that of ATP. Thus, it can be used to power the ATP synthesis from ADP. This is called substrate-level phosphorylation because the phosphate donor is a Substrate with high phosphoryl-transfer potential. PEP has high phosphoryl-transfer potential, pyruvate (ketone) is much more stable12 than enol form. Glycolysis summary •Inputs: •Glucose •2 NAD+ •2 ATP •4 ADP •2 Pi •Outputs: •2 pyruvate •2 NADH •2 ADP •4 ATP •2 ATP (net gain) Glucose + 2Pi + 2ADP + 2NAD+ 2pyruvate + 2ATP + 2NADH +2H+ + 2H2O 13 NAD regeneration 14 +O2 -O2 NAD regeneration 15 Transition reaction inputs and outputs from glucose •Inputs: •2 pyruvate •2 CoA •2 NAD+ •Outputs: •2 acetyl CoA •2 CO2 •2 NADH Pyruvate dehydrogenase Pyruvate + NAD+ + CoA Acetyl-CoA + CO2 + NADH Link between glycolysis and TCA cycle 16 S-CoA TCA Cycle 17 Critical steps Citrate synthase Oxidation 2nd oxidative decarboxylation decarboxylation isocitrate dehydrogenase a-ketoglutarate dehydrogenase 18 Enery-rich thioester compound Critical steps Succinyl CoA synthetase Oxidation Hydration Oxidation Oxaloacetate is regenerated for next cycle Energy is extracted in the form of FADH2 and NADH 19 Citric acid cycle inputs and outputs per glucose molecule •Inputs: •2 acetyl groups •6 NAD+ •2 FAD •2 GDP + 2 P •Outputs: •4 CO2 •6 NADH •2 FADH2 •2 GTP Extremely efficient: conserve 90% of energy available from oxidation of acetyl CoA Acetyl-CoA + 3NAD+ + FAD + GDP + Pi + 2H2O 2CO2 + 3NADH + FADH2 + GTP + 2H+ + CoA 20 Fatty acids Glucose Pyruvate Ketone bodies O CH3C-SCoA (2C) Acetate Amino Acids CoASH 4C NADH + H+ FADH2 Minimal TCA 6C NADH + H+ CO2 4C GTP GDP NADH + CO2 H+ 1 GTP 3 NADH +1 FADH2 10 ATP/cycle And releases two CO2 NOTE: 1 NADH 2.5 ATP; 1 FADH2 1.5 ATP; 1 GTP 1 ATP so get 1 + 7.5 + 1.5 = 10 ATP/cycle Will be revisited later in detail! Metabolism Harvesting electrons for the Electron transport chain 22 TCA Connections Acetyl CoA Amino Acid Synthesis Oxaloacetate Malate a-ketoglutarate Succinyl CoA Gluconeogenesis Heme Fatty Acid Synthesis Citrate Amino Acid and Neurotransmitter Synthesis 23