Products from Rocks
advertisement

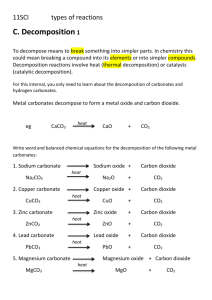
Products from Rocks C1a Limestone is mainly made from calcium carbonate CaCO3 Limestone used to make glass HEAT AT HIGH TEMPERATURE Powdered Limestone Sand Sodium carbonate Limestone used to make cement HEAT Powdered Limestone Powdered clay Limestone used to make Concrete MIX Cement powder Water Sand Crushed rock Thermal decomposition – breaking down a chemical by heating Thermal decomposition of limestone Heat Calcium Carbonate CaCO3 Calcium oxide + Carbon dioxide CaO + CO2 Limestone decomposes to form calcium oxide (quicklime) and carbon dioxide General equation for the thermal decomposition of a metal carbonate Metal carbonate Metal oxide + Carbon dioxide Quicklime + water Slaked lime Calcium oxide + water Calcium hydroxide CaO + H20 Ca(OH)2 Dissolve slaked lime (calcium hydroxide) in water Filter Produces limewater Lime water – used to test for carbon dioxide Calcium hydroxide + carbon dioxide Calcium carbonate + water Ca(OH)2 + CO2 CaO3 + H2O Mortar – slaked lime + sand + water Uses - holds building materials together How – Lime in mortar reacts with carbon dioxide in air producing calcium carbonate Very strong Cement - Limestone + clay Portland Cement – Limestone + clay + other minerals Uses – Modern house building How – Portland cement and sand mixed with water Left for a few days to set Concrete – Stones/crushed rocks + water + cement + sand Very strong – resists forces Reinforced concrete – Poured around steel rods or bars Glass – Powdered limestone + sand + sodium carbonate + strong heat Waterproof and light Available with different properties Metals found in Earths crust, mostly combined with other elements, often oxygen Metal ore – rock containing metal or metal compound Native state – some metals so unreactive they are found as the element naturally The reactivity series is the best way to extract a metal from its ore Metals more reactive than carbon cannot be extracted from their ores using carbon Many metals are found as oxides – combined with oxygen Heat metal oxide with carbon, carbon removes the oxygen from the metal oxide to produce carbon dioxide Metal oxide + Carbon Metal + Carbon dioxide We call the removal of oxygen in this way a reduction reaction Iron is extracted from iron ore by reducing it with carbon in a blast furnace Haematite – most common iron ore: mainly iron (III) oxide and sand Coke – reducing agent: mainly carbon Limestone – removes impurities C + O2 CO2 Hot air into blast furnace Coke burns Heats furnace Forms carbon dioxide gas CO2 + C 2CO Carbon dioxide reacts with coke Carbon monoxide gas formed Fe2O3 + 3CO 2Fe + 3CO2 Carbon monoxide reacts with iron oxide Reducing it to molten iron Flows to bottom of furnace Pig iron – produced from blast furnace Many impurities, mainly carbon Remove impurities from pig iron – get pure iron – very soft Metal that contains other elements - alloy Iron alloyed with other elements - steel Carbon steel – 0.03 – 1.5% carbon Cheapest steel Used – cars, knives, machinery, ships, containers, structural steel High carbon steel – lots of carbon – very strong but brittle Low carbon steel – soft and easily shaped, not as strong but less likely to shatter Mild steel – less than 0.1% carbon – easily shaped – mass production of cars Low-alloy steel – 1 – 5% other metals, e.g. nickel, chromium, manganese, vanadium, titanium, tungsten Low alloy nickel – Resistant to stretching forces long span bridges, bike chains, military armour plating. Low-alloy tungsten – good at high temperature High-speed tools High alloy steel – Chromium 12 – 15% Sometimes some nickel too Strong, chemically stable Stainless steel DO NOT RUST! Copper - very soft Bronze – copper and tin plus other elements, e.g. phosphorus Low friction properties Brass – Copper and zinc Hard Can be bent and shaped Smart alloys Shape memory alloys When deformed they return to their original shape when heated Shape memory alloys used in medicine – broken bones Dentistry - braces Transition metal – Good conductors of electricity and heat hard, tough and strong Malleable high melting points Copper extraction – Chemical – use sulfuric acid to produce copper sulfate solution Copper extraction – smelting – heat copper ore strongly in air crude copper Use impure copper as anodes in electrolysis cells 85% of copper produced like this New ways – bacteria, fungi, plants to extract copper Cheaper, environmentally friendly alternatives to extraction methods Aluminium and titanium useful as they resist corrosion Al and Ti expensive to extract from ores as requires lots of energy ££££££££££££ Al extraction – electrolysis Pass an electric current through molten Aluminium oxide at high temperatures Ti extraction – Displacement using sodium or magnesium Need to use electrolysis to produce these first Electrolysis – very expensive, lots of energy due to high temperatures and electricity needed Recycling Al is important Uses much less energy to produce same amount of recycled Al than extract it Crude oil – mixture of many different chemical compounds Not very useful Crude oil must be separated by distillation, into its different substances before it can be used. Distillation separates liquids with different boiling points Nearly all compounds in crude oil are made from atoms of hydrogen and carbon. HYDROCARBONS Most of the hydrocarbons in crude oil are ALKANES General chemical formula of an alkane CnH2n + 2 E.g. Methane CH4 (C = 1, H = (2 x 1+ 2) = 4) Alkanes – saturated hydrocarbons Contain as much hydrogen atoms as possible in their molecules Separate crude oil using fractional distillation Properties of each fraction depend on the size of the hydrocarbon molecules Short molecules – Lower boiling point High volatility Low viscosity Flammable Long molecules High boiling points Low volatility Viscous (thick) Smoky flame Crude oil separated in a fractioning column Temperature decreases going up the column Gases condense when they reach their boiling points Hydrocarbons with smaller molecules – lower boiling points – collect at the cool top of the tower Light crude oil – many smaller molecules Used as fuels More expensive than heavy crude oil Hydrocarbons burn in air they produce carbon dioxide and water Example: Propane + oxygen carbon dioxide + water C3H8 + 5O2 3CO2 + 4H2O Impurities in fuels may produce other substances which may be poisonous and cause pollution Sulfur dioxide – causes acid rain Most fuels contain some sulfur, which reacts with oxygen when burned Hydrocarbons in car engine Not enough oxygen inside car cylinders, so instead of all changing to carbon dioxide, produces carbon monoxide instead. Incomplete combustion Nitrogen oxides : High temperatures in cars cause N and O in air to react Poisonous Trigger asthma Acid rain Diesel cars – use larger molecule hydrocarbons Do not always burn completely Tiny particles are produced containing carbon and unburnt hydrocarbons Damaging when breathed in Some substances released when fuels are burnt dissolve in droplets of water in air. ACID RAIN GLOBAL WARMING Carbon dioxide greenhouse gas Reduces amount of heat lost by radiation GLOBAL DIMMING Particulates reflect sunlight back into space Catalytic convertors exhaust gases catalytic converter pass over transition metals arranged with large surface area carbon monoxide and nitrogen oxide react produce carbon dioxide and nitrogen reduces pollution Flue gas desulfurisation (FGD) Power stations – sulfur dioxide reacts with quicklime to cut pollution Gasohol Plants that make sugar produce ethanol by fermenting the sugar using yeast. Can use this by adding to petrol Less pollution – burns more cleanly Biodiesel Oilseed rape Plants take in carbon dioxide, even though they give it out when burnt Overall this cancels out Energy can be produced from rubbish in an incinerator Disadvantages – produces dioxins which may be dangerous