21-2-2011
advertisement

21-2-2011
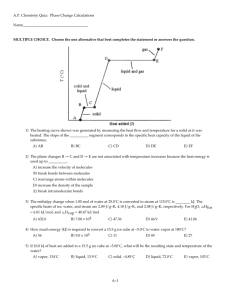
Clausius – Clapeyron Equation
• This equation is a relation between DHvap
and pressure at a certain Temperature.
• Example 1: Water has a vapor pressure of 24 mmHg at
25oC and a heat of vaporization of 40.7 kJ/mol. What is
the vapor pressure of water at 67oC?
• Solution: Simply use the Clausius-Clapeyron Equation
to figure out the vapor pressure. We have to be a bit
careful about the units of R: the units we're using are kJ,
so R = 8.31x10-3 kJ/mol K.
ln(P2/P1) = -DHvap/R * (1/T2- 1/T1)
ln(P2/24) = - 40.7 kJ/8.31x10-3 kJ/mol K *(1/340- 1/298)
ln(P2/24) = 2.03
P2/24 = 7.62
P2 = 182 mmHg
• Example 2 :An unknown liquid has a vapor
pressure of 88 mmHg at 45oC and 39 mm Hg at
25oC. What is its heat of vaporization ?
• Solution :use the Clausius-Clapeyron Equation.
Here, the only thing we don't know is DHvap
ln(88/39( = )DHvap/8.31x10-3){(1/318) – (1/298)}
DHvap = 32.0 kJ
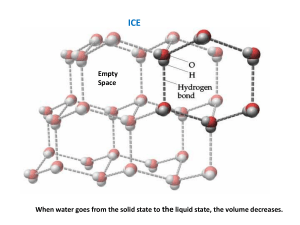
Liquid-solid equilibrium
• A solid can be transformed into a liquid at
a specific temperature called the melting
point.
• Melting occurs as temperature increases
the kinetic energy of the molecules and
thus make them move around.
• The melting point (or freezing point) is the
temperature at which solid and liquid
phases coexist in equilibrium.
Normal melting or freezing points
• The temperature at which both solid and
liquid phases coexist at equilibrium at 1
atm is called the normal melting point
(normal freezing point).
Ice
Water
Molar heat of fusion (DHfus)
• The energy required to melt 1 mole of a solid is
called the molar heat of fusion (DHfus).
• The molar heat of fusion is definitely smaller
than the molar heat of vaporization since
vaporization requires complete removal of the
molecules from the surface while fusion only
requires rearrangement from solid to liquid.
Solid-vapor equilibrium
• Solids can undergo direct evaporation, and
thereby solids are said to have a vapor
pressure.
Ice
vapor
Sublimation: is a process in which molecules can
go directly from the solid to the vapor phase.
The reverse process is called “deposition”.
Naphthalene (mothballs) and I2 are examples.
Molar heat of sublimation (DHsub)
• The energy required to sublime 1 mole of a solid
is called the molar heat of sublimation.
DHsub = DHfus + DHvap
• This is, in fact, a manifestation of Hess’s law,
where the amount of energy needed to
transform a solid to vapor is the same whether
we go directly or in steps by first transforming
the solid to a liquid and then transforming the
liquid to vapor.
Example
Find the energy in kJ necessary to melt 1.00 g of
ice. DHfus of ice = 5.98 kJ/mol
Solution
Melting of one mole of ice requires 5.98 kJ,
therefore, find moles of ice present in 1.00 g and
find energy required.
Energy needed = DHfus * Number of moles
Energy needed = 5.98 kJ/mol * {1.00 g/(18.0
g/mol)} = 0.332 kJ
Heating and Cooling Curves
When heat is added to a solid its
temperature will rise till the melting point is
reached where the temperature stays
constants till the entire solid is converted
to liquid. When extra heat is added to the
system, the temperature of the liquid starts
to rise till the boiling point is reached
where the temperature stays constant till
all the liquid is converted to vapor. This
can be represented by a heating curve as
below:
Example
Find the energy needed to convert 36.0 g of ice at
-23 oC into vapor at 120 oC. DHfus = 5.98 kJ/mol
and DHvap = 44.0 kJ/mol, specific heat of water is
4.184 J g-1 oC-1, specific heat of ice is 2.06 J g-1
oC-1, and specific heat of steam is 1.99 J g-1 oC-1.
Solution
Number of moles = 36.0/18.0 = 2.00 moles
Following the heating curve above, ice should first
be
1. It will require energy to be converted to ice at 0 oC
(heat capacity 1)
2. It will require the heat of fusion to convert to liquid at
0 oC
3. It will need energy to be converted to liquid at 100
oC, boiling temperature for water (heat capacity 2)
4. It will require the heat of vaporization to be
converted to water vapor at 100 oC
5. It will require energy to raise the temperature of the
vapor to 120 oC (heat capacity 3)
Therefore, five energy terms should be summed
together. Steps 1, 3, and five can be summed
together as heat capacity term:
Energy required = (heat capacity term) + number
of moles *(DHfus + DHvap)
Energy required = {36.0 g * 0.00206 kJ g-1 oC-1 {0
– (-23)} oC + 36.0 g * 0.004184 kJ g-1 oC-1 {100 –
(0)} oC + 36.0 g * 0.00199 kJ g-1 oC-1 {120 –
(100)} oC} + 2.00 mol * (5.98 + 44.0)kJ/mol =
118.2 kJ
Phase Diagrams
• A phase diagram is a graph that
represents and summarizes conditions
under which a substance exist as liquid,
solid, or gas.
• We will only look at the phase diagrams of
water and carbon dioxide
A phase diagram
Triple point
• The triple point is the point at which all
three phases coexist at equilibrium.
• For water, the triple point occurs at 0.01
oC, and 0.006 atm.
Water phase diagram
Example
Describe any changes in the phases present
when water is:
1. Kept at 0 oC while pressure is increased
from that at point 1 to that at point 5.
2. Kept at 1 atm while the temperature is
increased from that at point 6 to that at
point 9.
CO2 Phase diagram
Answer the following problems:
• 1-3, 7, 9, 11-20, 22, 27-29, 31, 54, 56-60,
62, 65, 66, 69, 71-75, 80, 86, 99.