Bonding and Naming WS 4 Supplement for Extra
advertisement
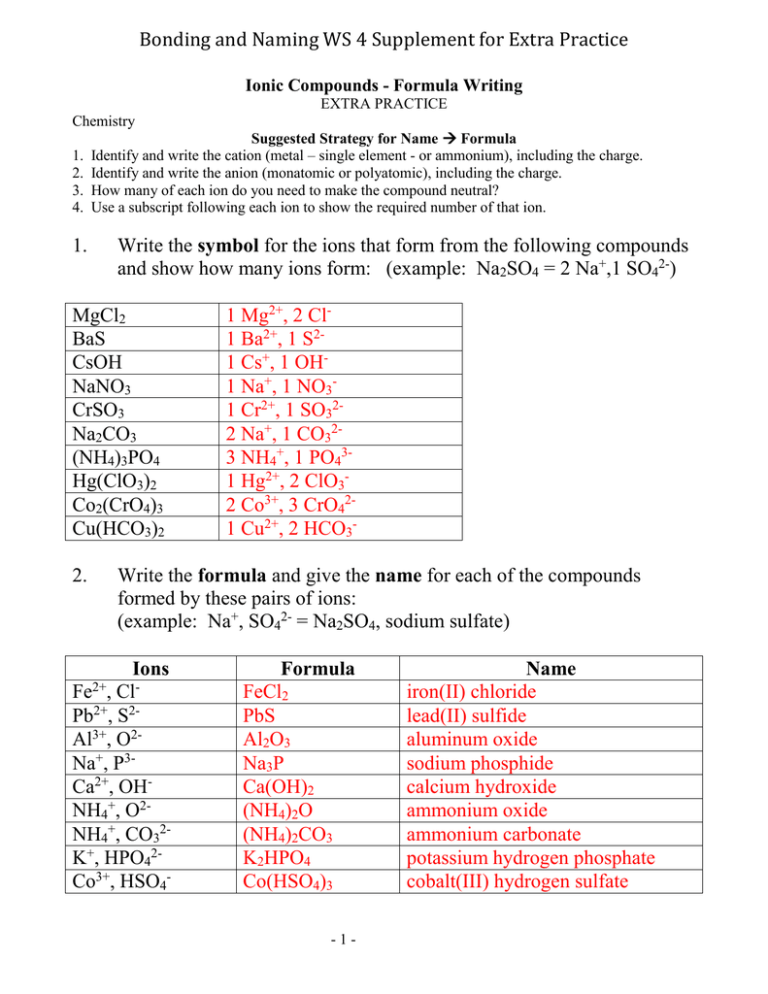
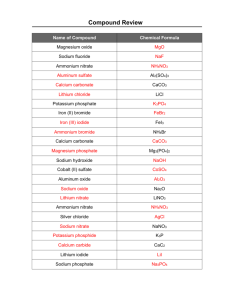
Bonding and Naming WS 4 Supplement for Extra Practice Ionic Compounds - Formula Writing EXTRA PRACTICE Chemistry 1. 2. 3. 4. Suggested Strategy for Name Formula Identify and write the cation (metal – single element - or ammonium), including the charge. Identify and write the anion (monatomic or polyatomic), including the charge. How many of each ion do you need to make the compound neutral? Use a subscript following each ion to show the required number of that ion. 1. Write the symbol for the ions that form from the following compounds and show how many ions form: (example: Na2SO4 = 2 Na+,1 SO42-) MgCl2 BaS CsOH NaNO3 CrSO3 Na2CO3 (NH4)3PO4 Hg(ClO3)2 Co2(CrO4)3 Cu(HCO3)2 2. 1 Mg2+, 2 Cl1 Ba2+, 1 S21 Cs+, 1 OH1 Na+, 1 NO31 Cr2+, 1 SO322 Na+, 1 CO323 NH4+, 1 PO431 Hg2+, 2 ClO32 Co3+, 3 CrO421 Cu2+, 2 HCO3- Write the formula and give the name for each of the compounds formed by these pairs of ions: (example: Na+, SO42- = Na2SO4, sodium sulfate) Ions Fe , ClPb2+, S2Al3+, O2Na+, P3Ca2+, OHNH4+, O2NH4+, CO32K+, HPO42Co3+, HSO42+ Formula FeCl2 PbS Al2O3 Na3P Ca(OH)2 (NH4)2O (NH4)2CO3 K2HPO4 Co(HSO4)3 -1- Name iron(II) chloride lead(II) sulfide aluminum oxide sodium phosphide calcium hydroxide ammonium oxide ammonium carbonate potassium hydrogen phosphate cobalt(III) hydrogen sulfate Bonding and Naming WS 4 Supplement for Extra Practice 3. Write the formula for each of the following compounds: (example: sodium sulfate = Na2SO4) Name Formula silver oxide barium fluoride tin(IV) sulfide magnesium nitride sodium carbonate aluminum hydroxide ammonium nitrate cobalt(III) sulfate copper(I) phosphate strontium acetate iron(III) hydrogen sulfate lead(II) oxide magnesium cyanide potassium hydrogen phosphate chromium(II) oxalate 4. Ag2O BaF2 SnS2 Mg3N2 Na2CO3 Al(OH)3 NH4NO3 Co2(SO4)3 Cu3PO4 Sr(C2H3H2)2 Fe(HSO4)3 PbO Mg(CN)2 K2HPO4 CrC2O4 Write the name for each of the following ionic compounds: (example: Na2SO4 = sodium sulfate) Formula RbI Al2O3 PbS Sn(OH)2 Na2CO3 SrCr2O7 (NH4)2 SO4 Mg(ClO2) 2 Al(HCO3)3 Co2(CrO4)3 Name rubidium iodide aluminum oxide lead(II) sulfide tin(II) hydroxide sodium carbonate strontium dichromate ammonium sulfate magnesium chlorite aluminum hydrogen carbonate cobalt(III) chromate -2-