Document
advertisement

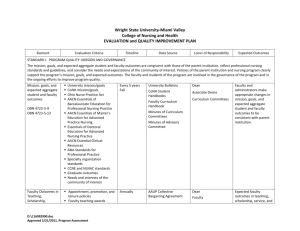
ESSENTIAL NMR EXPERIMENTS FOR EVERY ORGANIC CHEMIST A presentation by Dr. M. Sales in the Laboratories of Prof. A. B. Charette 10 January 2005 General Outline 1. Introduction to information that can be obtained from NMR spectroscopy 2. A Diels-Alder adduct is used as an example to illustrate The various NMR experiments that can be obtained 2.1-2.3 Examples of C,H (heteronuclear) correlation experiments 2.4, 2.5 and 2.10 Examples of H,H (homonuclear) correlation experiments 2.6-2.9 Examples of NOE and chemical exchange experiments 3. A brief application of experiments on a precursor substrate 4. Recommended resources for a detailed INTRODUCTION to NMR Theory and Application Problems, problems Question Design Question substrate, reagents molar equivalents rxn time, rxn temperature Design Execution reaction-setup order of addition of reagents Execution Process reaction workup purification Process identify product/s ratio of products relative configuration scalar coupling dipolar coupling (NOE or chemical exchange) Appropriate 1D / 2D NMR expt signal to noise ratio Resolution Fourier transforms Window functions Zerofilling Analysis Result Answer The complexity of the questions asked is only limited by the ability to analyze the 'complex' results. 1. What information can NMR spectroscopy provide Structural Theory NMR equivalent Qualitative composition Choice of nucleus / isotope Quantitative composition Signal intensities / integration Functional groups Chemical shifts Connectivity J (scalar) couplings Spatial relationship of atoms NOE (distance) J couplings (dihedral angle) Dynamics Chemical exchange, lineshape broadening 1.1 Functional group information from Chemical Shift 1.2 Connectivity information from scalar coupling 1J 2J H C HC HC, 3J H HC H C 2J HH and 2J 3J 5J HH , HMQC (Heteronuclear Multiple Quantum Coherence) 3J HH, HH…… H HH 4J C HH, C H H H H C H H C H H HMBC (Heteronuclear Multiple Bond Correlation) 2D COSY (Correlation SpectroscopY) Homodecoupling, 1D selective COSY 2D TOCSY (TOtal Correlation SpectroscopY) 1D selective TOCSY 1.3 Spatial Relationships of atoms from scalar couplings The Karplus equation relates 3JHH with the dihedral angle. 3J HH values are obtained from routine H NMR spectra and homodecoupling experiments. C. Altona et al Magn. Res. Chem. 1994, 32, 670 O H O O OMOM 4a 4 8a O OH Coupling constant analysis assigns the 4,4a-syn/anti relationship OH 3" 3 4 1' S N OMOM N S S 4a S O 4 H 4,4a-syn N O R O 216 N H J4-4a = 3-4 Hz 4" S 215 8a O H O O OMOM 4a 4 8a S 4,4a-anti O H O S S 217 8a 4a 4 H O R J4-4a = 8-11 Hz 1.4 Spatial Relationships of atoms from NOE H CO2CH3 H N H o CO2CH3 150-170 C Bz Bz N H H H3CO2C NOE Bz Typical usage of NOE relationships in literature to assign relative stereochemistry Arakawa, Y. et al Chem. Pharm. Bull. 2003, 51, 1015-1020 1.5 Investigation of Dynamic processes by observation of Chemical Exchange 2.9ppm CH3 Line-broadening and coalescence of signals are routine methods to investigate Dynamics in moleculer systems. However, 1D and 2D EXSY (EXchange SpectroscopY) methods can indicate chemical exchange before line broadening occurs 2.8ppm CH3 O CH3 N H CH3 2.8ppm CH3 Perrin, C. L. and Dwyer T. J. Chem. Rev. 1990, 90, 935-967 2.9ppm CH3 2D EXSY of N,N-dimethylformamide 2. DA derivative used in NMR study O Diels-Alder N Ph + N O N Ph N O O Functioal Group Interconversion Ph N OBn O O OBn O DA derivative Examples of the following NMR experiments of the DA derivative will be presented : 13C NMR DEPT HMQC HMBC 1H NMR 2D COSY 2D TOCSY 2D NOESY 1D NOE 2D ROESY homodecoupling 1D selective COSY 1D EXSY 2.1 C-13 and DEPT 7 O Ph 1 N 8 4 3 6 5 2' CH2 CH 2" CH3 OBn 1' 2 OBn 1" methine methylene methyl doublet (d) triplet (t) quartet (q) The d,t and q refers to the 13C multiplicity with respect to C31 H33 N O3 couplings from attached (geminal) protons. 7 CO 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 7 O Ph 1 N 8 4 5 2' 2" OBn 1' 3 4xAr d 2.1 C-13 and DEPT 6 2 Ar = one aromatic carbon # = 2xAr d OBn 1" C31 H33 N O3 # # * = Ar # d 2 x Ar s # 4 d Ar s 3 d * * * 2 x Ar d 139 138 137 136 135 134 133 132 131 130 129 128 127 126 7 O Ph 1 N 8 4 3 6 5 2' 2.1 C-13 and DEPT 2" OBn 1' 2 OBn 1" C31 H33 N O3 2” 6 1’ 2 5 7 2’ 1” 2 x CH2Ph 70 60 50 40 30 20 2.2 HMQC 7 O Ph 1 N 8 4 3 6 5 2' 2" OBn 1' 2 4 3 2 5 1’ 2’ OBn 1" H C 1” 2” 7 O Ph 1 N 8 4 2.2 HMQC 6 5 2' 3 2" OBn 1' 2 1” OBn 1" 2” H C o-Ar 1’ 5 2’ 3 4 2 6 2.3 HMBC 7 O Ph 1 N 8 6 5 2' 4 2" OBn 1' 3 2 OBn 1" H C H C C C D C A C Regions A, B, C and D are expanded on next slide. B 7 O Ph 1 N 8 4 2.3 HMBC 6 5 2' 2" OBn 1' 3 o-Ar (Bn) 2 OBn Unambiguous assignment of ortho-Ar to the benzylic moeity attached to C-1”. 1" H C C 6 CO H C 2 CH2Ph (1”) C C CH2Ph (2”) Region B Region A C-1” C-2’ CH2Ph (1”) C-5 2” 2” C-2” CH2Ph (2”) CH2Ph (1”) C-1’ 1” 1” C-2 CH2Ph (2”) 2” 2” Region C Unambiguous assignment of both CH2Ph is achieved from analysis of HMBC spectrum C-6 Region D 1” 1” 2.4 COSY 7 O Ph 6 1 N 8 5 2' 4 3 7 2" OBn 1' 2 OBn 1" 5 H H H H 6 2 3 4 2.4 COSY 7 O Ph 1 N 8 4 2 6 5 2' 2 2" OBn 1’ 1" 5 H H 1’ 5 OBn 1' 3 6 H H 6 2 2.4 COSY 7 O Ph 1 N 8 4 6 2’ 5 2' OBn 1' 3 2" 2 1’ OBn 1" 1” H H H H 2” 2” 1” 2.4.1 Spin system deduced from COSY 6(4.18) 7(1.27) 2"(3.35) 7 O Ph 1 N 8 4 3 4(6.37) 5(2.92) 2'(2.49) 6 5 2' 2"(3.17) 2" OBn 1' 2 OBn 1" 1"(3.39) 3(6.28) geminal J (2 bond coupling) according to HMQC vicinal J (3 bond coupling) 2(4.56) 1'(2.56) 1"(2.96) 2.5 Homodecoupling Reference (Irradiate 9 ppm) 2 (4.56 ppm) 2.6, 5.5 Hz 4 (6.37 ppm) decoupled (Irradiate 6.37 ppm) 6.8 6.6 6.4 6.2 6.0 5.8 5.6 5.4 5.2 5.0 4.8 4.6 4.4 4.2 7 O Ph 1 N 8 4 3 reference J 2-4 = 0.5 Hz 6 5 2' 2" OBn 1' 2 OBn 1" J 2-3 = 5.5 Hz J 2-1’ = 2.6 Hz decoupled 4.60 4.55 4.50 4.45 2.5 Homodecoupling Reference (Irradiate 9 ppm) 7 O Ph 1 N 8 6 5 2' 4 3 2" OBn 1' 2 3 (6.28 ppm) OBn 1’ (2.56 pm) 2 (4.56 ppm) 1.5, 7 Hz 4.7, 9.5, 10 Hz 1" Decoupled (Irradiate 4.56 ppm) 6.6 6.4 6.2 6.0 5.8 5.6 5.4 5.2 5.0 4.8 4.6 4.4 J 2-3 = 5.5 Hz J 1’-2 = 2.6 Hz J 3-5 = 1.5 Hz J 3-4 = 7 Hz J 1’-1” = 4.7 Hz J 1’-1” = 9.5 Hz J 1’-2’ = 10 Hz 6.45 6.40 6.35 6.30 6.25 2.70 2.65 4.2 4.0 3.8 3.6 3.4 3.2 3.0 2.8 2.6 2.4 2.2 reference decoupled 2.60 2.55 2.50 2.45 2.5.1 Analysis of coupling constants 6(4.18) 6.1 Hz 6.5 Hz O Ph 1 N 8 4 3 4(6.37) 1.8 Hz 5(2.92) 6 8.5 Hz 2 2"(3.17) 2" OBn 1' 8.9 Hz 2'(2.49) 1.5 Hz 5 2' 2"(3.35) 5.8 Hz 1.8 Hz 7 7(1.27) 7 Hz 10 Hz OBn 1" 1"(3.39) 0.5 Hz 4.7 Hz 3(6.28) 4 possible relative configurations where dihedral angle between HC-1’ and HC-2’ is = 0 º. (J 1’-2’= 10 Hz) 5.5 Hz 2(4.56) O Ph N 1"(2.96) O N OBn Ph O N OBn A 9.5 Hz 1'(2.56) 10 Hz O Ph 2.6 Hz OBn OBn B OBn Ph N OBn C D OBn OBn 2.6 2D NOESY O Ph 7 6 1 N 8 3 5 4 3 1’ 2’ 4 6 2' 1' 2" 2 1" 7 5 OBn 1’ 2’ OBn Integration of NOE crosspeaks : Reference = Diagonal of protons 1’ and 2’ Integrate for 2.00 4 3 6 5 7 -1% NOE -4% NOE -3% NOE 2.6.1 Assignment of benzylic protons with the aid of NOE crosspeaks Note: No scalar couplings between 1” or 2” to any of the benzylic were observed in the COSY or homodecoupling experiments. Therefore NOE/dipolar interactions are used to assign benzylic protons O Ph 7 1’/2’-2 -4% 6 2” 1” 5 1’ 2’ 1’/2’-6 -7% 5 4 3 2 1” 2” 6 1 N 8 CH2Ph CH2Ph 2' 1' 2 1" 2" OBn OBn CH2Ph (1”) CH2Ph (2”) Note: The combined NOE between 1’,2’-6 of -7% offers further support for assigned relative configuration 1”-CH2Ph -3% 2”-CH2Ph -3% 1”-CH2Ph -2% 2”-CH2Ph -3% 5-6 -5% 1”-2 -2% 2.6.2 Chemical exchange crosspeaks observed in 2-D NOESY O O N N Z OBn OBn OBn E OBn E/Z isomerism of 3o amide 2.6.2 Chemical exchange crosspeaks observed in 2-D NOESY 2 2 (Z) These expansions of the 2-D NOESY spectrum clearly indicate that the crosspeaks correlate the proton resonances of the major conformer (E) to those of the minor conformer (Z). This establsihes the identity of the minor species in the H NMR spectrum as a conformer of the major compound and not a contaminant. (E) 2 (E) 2 (Z) 7 (E) 7 (Z) 7 (Z) 6 (E) 2” (E) 2” (Z) 7 (E) 6 (Z) 6 (E) 6 (Z) 2” (Z) 2” (E) 2.6.3 Further support for the assigment of o-Ar (Bn) via analysis of NOE crosspeaks O N H H -0.5% OBn O H H H -1% and -1.5% CH2Ph (1”) 2 o-Ar (PhCON) region expanded -0.5% -1% -1.5% O Ph 7 8 2.7 1D NOE 6 1 N 5 4 3 2' 1' 2 1" 2" OBn OBn 7 4 1.5% 3 0.5% 6 4% 5 3% 4 Irradiation Frequency = 5 8% 2 3% 3 40% 7 (Z) 3 4 34% 7.0 6.5 2 9% 6.0 5.5 5.0 5 5% 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 O Ph 7 8 2.8 2D ROESY 6 1 N 5 4 3 2' 1' 2 1" 2" OBn OBn n0 = Larmor frequency of precessing proton nucleus tc = correlation time which is dependant on solvent viscosity and moleculer weight Imax/Io represents relative maximum crosspeak signal intensity 2D NOESY tc → maximum 20%, the case for small molecules tc → 0 maximum 50%, the case for proteins o x tc = 0 the crosspeak intensitiy = 0 2D ROESY tc → 0 maximum 20% tc → maximum 34% Useful for molecules of intermediate molecule weight O Ph 7 6 1 N 8 3 2.8 2D ROESY 5 4 2' 1' 2 1" 2" OBn OBn 4 Chemical exchange crosspeaks observed in 2D ROESY ROE crosspeaks detected between methyl proton 7 to protons 4 and 3 support assigned relative configuration 3 7 E 7 Z 7 0.15 % ROE 0.3 % ROE Integration of NOE crosspeaks : Reference = Diagonal of protons 1’ and 2’ Integrate for 2.00 7 E 7 Z O Ph 7 6 1 N 8 2.9 TOCSY 5 4 2' 1' 3 2" 2 1" OBn 1” 2” 5 1” 1’ 2’ 2” OBn B A H H H H regions A and B are expanded on next slide H O Ph 7 1 N 8 2.9 TOCSY 6 5 4 3 2' 1' 2" 2 1" 1” 2” H OBn H H H OBn 2” H 1” 5 4 1’ 2’ 7 1’ 2’ 1’ 2’ 5 1” 1” 5 2” 1” 2” 2” 1” 2” 2 Region A Region B 3 2.10 1D EXSY experiment 1D EXSY (EXchange SpectroscopY) uses the same pulse sequence as the 1D NOESY except that the mixing time has been optimised to observe chemical exchange. Reference (1H NMR) O Ph 7 6 1 N 8 5 4 3 2' 1' 2 1" 2" OBn Expt 1 (1D EXSY) OBn 2 (Z) Irradiation Frequency = 2 (E) Expt 2 (1D EXSY) 7 (E) 8.0 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 7 (Z) 1.0 0.5 3.1 Chemical exchange I N Ph N MeI I N N O N 7 Ph 6 5 N OH- N 4 OBn 3 OBn 2 Z OBn OBn OBn E OBn OBn OBn H NMR revealed two conformers Z : E in ratio 1 : 2 7 (E) 4 (E), 4 (Z) 2 (E) 3 (Z) 9.0 8.5 8.0 7.5 7.0 6.5 3 (E) 6.0 7 (Z) 2 (Z) 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 3.2 1D selective COSY used for assignment of proton resonances 7 1 6 N 5 N I 4 (E), 4 (Z) 4 3 2 Z H H 2 (E) OBn 3 (Z) OBn H 7 (E) 3 (E) 2 (Z) 7 (Z) H 3 (Z) 2 (Z) Irradiation Frequency = 2 (E) 3 (E) 9.0 8.5 8.0 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 1.0 0.5 3.3 1D EXSY experiment I 7 1 6 N 5 N 4 3 2 Z OBn OBn These 1D EXSY experiments provide evidence that the two species observed in the H NMR spectrum are conformers. 3 (Z) 3 (E) 3 (Z) 3 (E) Irradiation Frequency = 2 (Z) 2 (E) 7 (E) 9.0 8.5 8.0 7.5 7.0 6.5 6.0 5.5 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 7 (Z) 1.0 0.5 4. Recommended resources for a detailed INTRODUCTION to NMR Theory and Application H. Friebolin Basic One- and Two-Dimensional NMR Sectroscopy 3rd Edition 1998 Wiley-VCH Croasmun W. R., Carlson R. M. K. Two-Dimensional NMR Spectroscopy – Applications for Chemists and Biochemists 2nd Edition 1994 VCH Publishers Sanders J. K. M. and Hunter B. K. Modern NMR spectroscopy – A Guide for Chemists 2nd Edition 1993 Oxford University Press Derome A. E. Modern NMR techniques for chemistry research 1st Edition 1987 Pergamon press Braun S., Kalinowski H.-O., Berger S. 150 and More Basc NMR Experiments – A Practical Course 2nd Edition 1998 Wiley VCH Publishers